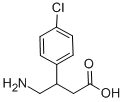
바클로펜
|
|
바클로펜 속성
- 녹는점
- 208-210°C
- 끓는 점
- 364.3±32.0 °C(Predicted)
- 밀도
- 1.2069 (rough estimate)
- 굴절률
- 1.5500 (estimate)
- 저장 조건
- 2-8°C
- 용해도
- 1M HCl: 50mg/mL
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- pKa 3.87±0.1(H2O) (Uncertain)
- 색상
- 흰색에서 아주 희미한 노란색
- 수용성
- 묽은 NaOH 또는 묽은 HCl에 용해됩니다. pH 7.6에서 약 4mg/ml로 물에 용해됩니다.
- Merck
- 14,937
- 안정성
- 흡습성
- InChI
- InChI=1S/C10H12ClNO2/c11-9-3-1-7(2-4-9)8(6-12)5-10(13)14/h1-4,8H,5-6,12H2,(H,13,14)
- InChIKey
- KPYSYYIEGFHWSV-UHFFFAOYSA-N
- SMILES
- C1(C=CC(Cl)=CC=1)C(CN)CC(=O)O
- CAS 데이터베이스
- 1134-47-0(CAS DataBase Reference)
- NIST
- Baclofen(1134-47-0)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 61-25-36/37/38-42/43-20/21/22 | ||
| 안전지침서 | 53-22-36/37/39-45-52-36-26 | ||
| 유엔번호(UN No.) | UN 2811 6.1/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | MW5084200 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 2922492050 | ||
| 독성 | LD50 in male mice, rats (mg/kg): 45, 78 i.v.; 103, 115 s.c.; 200, 145 orally (Tadokoro) | ||
| 기존화학 물질 | KE-01454 |
바클로펜 C화학적 특성, 용도, 생산
화학적 성질
Off-White Solid용도
Baclofen may be used as a pharmaceutical secondary reference standard for the determination of the analyte in plasma samples by liquid chromatography tandem mass spectrometry and tablet formulations by UV spectroscopy, respectively.정의
ChEBI: A monocarboxylic acid that is butanoic acid substituted by an amino group at position 4 and a 4-chlorophenyl group at position 3. It acts as a central nervous system depressant, GABA agonist and muscle relaxant.Biological Functions
Baclofen (Lioresal) is the parachlorophenol analogue of the naturally occurring neurotransmitter γ-aminobutyric acid (GABA).일반 설명
Odorless or practically odorless white to off-white crystalline powder.공기와 물의 반응
Insoluble in water.반응 프로필
Baclofen is an amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.화재위험
Flash point data for Baclofen are not available. Baclofen is probably combustible.생물학적 활성
Selective GABA B receptor agonist. Skeletal muscle relaxant.Mechanism of action
Baclofen appears to affect the neuromuscular axis by acting directly on sensory afferents, γ-motor neurons, and collateral neurons in the spinal cord to inhibit both monosynaptic and polysynaptic reflexes. The principal effect is to reduce the release of excitatory neurotransmitters by activation of presynaptic GABAB receptors. This seems to involve a G protein and second-messenger link that either increases K+ conductance or decreases Ca++ conductance.Clinical Use
Baclofen is an agent of choice for treating spinal spasticity and spasticity associated with multiple sclerosis. It is not useful for treating spasticity of supraspinal origin. Doses should be increased gradually to a maximum of 100 to 150 mg per day, divided into four doses.부작용
Side effects are not a major problem, and they can be minimized by graduated dosage increases.They include lassitude, slight nausea, and mental disturbances (in including confusion, euphoria, and depression). The drowsiness is less pronounced than that produced by diazepam—an important therapeutic advantage. Hypotension has been noted, particularly following overdose. Elderly patients and patients with multiple sclerosis may require lower doses and may display increased sensitivity to the central side effects. Baclofen may increase the frequency of seizures in epileptics.Safety Profile
Poison by ingestion,subcutaneous and intravenous routes. Human systemiceffects by ingestion: blood pressure lowering, coma,muscle weakness, pulse rate decrease, respiratorydepression. When heated to decomposition it emits toxicfumes of Cl-신진 대사
Baclofen is rapidly and effectively absorbed after oral administration. It is lipophilic and able to penetrate the blood-brain barrier.Approximately 35% of the drug is excreted unchanged in the urine and feces.바클로펜 준비 용품 및 원자재
원자재
준비 용품
바클로펜 공급 업체
글로벌( 540)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Joyochem Co.,Ltd | +86-0531-82687558 +8613290333633 |
sales@joyochem.com | China | 42 | 58 |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 |
mack@tongyon.com | China | 2996 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 |
market02@senwayer.com | China | 874 | 58 |
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 |
ziboweibinmaoyi@163.com | China | 100 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 |
sales8@anhuiyiao.com | China | 253 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 |
Sara@xmwonderfulbio.com | China | 305 | 58 |
| Jinan Million Pharmaceutical Co., Ltd | +86-531-68659554 +8613031714605 |
info@millionpharm.com | China | 154 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 |
nova@sh-teruiop.com | China | 525 | 58 |
바클로펜 관련 검색:
트리틸클로라이드 부틸디페닐클로로실란 감마-아미노-N-부티르 산 바클로펜
Chloromethyl butyrate
CHLOROPHENYLSILANE 97
4-Phenylbutyric acid
(R)-Baclofen
BACLOFEN (-), [BUTYL-4-14C]
(S)-Baclofen,S(+)-Baclofen
BACLOFEN IMPURITY A
R(+)-Baclofen.Hydrochloride
METHYL 3-(4-CHLOROPHENYL)-2-METHOXYCARBONYL-4-NITROBUTANOATE
1-[(TERT-BUTYL)OXYCARBONYL]-4-(4-CHLOROPHENYL)PYRROLINE-3-CARBOXYLIC ACID
(2R,3S)-BETA-P-CHLOROPHENYLGLUTAMIC ACID
Trans (+/-) 4-(4-Chlorophenyl)Pyrrolidine-3-Methylcarboxylate Hydrochloride
4-(4-CHLOROPHENYL)PYRROLIDINE-3-METHYLCARBOXYLATE HYDROCHLORIDE
FMOC-BACLOFIN