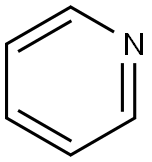
피리딘
|
|
피리딘 속성
- 녹는점
- -42 °C (lit.)
- 끓는 점
- 115 °C (lit.)
- 밀도
- 0.978 g/mL at 25 °C (lit.)
- 증기 밀도
- 2.72 (vs air)
- 증기압
- 23.8 mm Hg ( 25 °C)
- FEMA
- 2966 | PYRIDINE
- 굴절률
- n
20/D 1.509(lit.)
- 인화점
- 68 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 물에 용해됨
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 5.25(at 25℃)
- 색상
- 무색의
- 냄새
- 0.23~1.9ppm(평균 = 0.66ppm)에서 메스꺼운 냄새가 감지됩니다.
- 상대극성
- 0.302
- 수소이온지수(pH)
- 8.81 (H2O, 20℃)
- 폭발한계
- 12.4%
- Odor Threshold
- 0.063ppm
- ?? ??
- 비린내
- 수용성
- 혼용 가능
- 어는점
- -42℃
- 최대 파장(λmax)
- λ: 305 nm Amax: 1.00
λ: 315 nm Amax: 0.15
λ: 335 nm Amax: 0.02
λ: 350-400 nm Amax: 0.01
- Merck
- 14,7970
- BRN
- 103233
- Henry's Law Constant
- 18.4 at 30 °C (headspace-GC, Chaintreau et al., 1995)
- Dielectric constant
- 12.5(20℃)
- 노출 한도
- TLV-TWA 5 ppm (~15 mg/m3) (ACGIH, MSHA,and OSHA); STEL 10 ppm (ACGIH), IDLH 3600 ppm (NIOSH).
- 안정성
- 안정적인. 가연성. 강한 산화제, 강산과 호환되지 않습니다.
- InChIKey
- JUJWROOIHBZHMG-UHFFFAOYSA-N
- LogP
- 0.64 at 20℃
- CAS 데이터베이스
- 110-86-1(CAS DataBase Reference)
- IARC
- 2B (Vol. 77, 119) 2019
- NIST
- Pyridine(110-86-1)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,F,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-20/21/22-39/23/24/25-23/24/25-52-36/38 | ||
| 안전지침서 | 36/37/39-38-45-61-28A-26-28-24/25-22-36/37-16-7 | ||
| 유엔번호(UN No.) | UN 1282 3/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | UR8400000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 482 °C | ||
| 위험 참고 사항 | Highly Flammable/Harmful | ||
| TSCA | Yes | ||
| HS 번호 | 2933 31 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 110-86-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 1.58 g/kg (Smyth) | ||
| IDLA | 1,000 ppm | ||
| 기존화학 물질 | KE-29929 |
피리딘 C화학적 특성, 용도, 생산
물성
약한 염기성을 가지고 있으므로 산에는 염을 만들며 녹는다. 물 ·에탄올 ·에테르와 섞인다. 콜타르를 묽은 황산으로 처리하면 수용액이 되어 분리된다.용도
피리딘은 다양한 공업적 용도로 사용되는 화합물이다. 주요한 분야로는 제초제와 고엽제가 있다. 파라쾃(paraquat)은 피리딘으로부터 합성되는 초강력 제초제다. 아예 환원시켜서 비방향족 헤테로고리 화합물인 피페리딘을 합성하는데 사용되기도 한다. 그 외에 드물게 유기용매로 사용되기도 하고, 유기금속화학분야에서 유용한 고부가가치 화합물을 합성하는데 리간드로 이용되기도 한다. 여담이지만 니코틴 분자를 구성하는 주요 파트를 이루기도 한다. 오각형 비방향족 헤테로고리 화합물인 N-메틸피롤리딘과 연결된 형태가 바로 니코틴이다. 파란색이 피리딘 파트.용도
고무나 도료의 용제로 사용되며, 합성원료 ·분석시약으로도 사용된다.화학적 성질
Pyridine is a weak base (pKa= 5.25); a 0.2 M solution has a pH of 8.5 (HSDB 1988). Its carbon atoms are deactivated towards electrophilic substitution. This is especially true in acidic media, where salts form at the nitrogen. It does, however, readily undergo nucleophilic substitution, preferentially at the C-2 and also at the C-4 position (Jori et al 1983). Being a tertiary amine, pyridine reacts with alkylating agents to form quaternary salts (Santodonato et al 1985). Because of its reduced capacity to donate electrons, it is more resistant to oxidation than benzene. Oxidation with peroxy acids forms pyridine N-oxide which is then capable of undergoing electrophilic substitution (Jori et al 1983). Pyridine reacts violently with a number of compounds, including nitric acid, sulfuric acid, maleic anhydride, perchromate, beta-propiolactone and chlorosulfonic acid. Thermal decomposition can liberate cyanides (Gehring 1983). Both the pyridinium ion and pyridine itself are readily reduced to the commercially important compound, piperidine (Jori et al 1983).물리적 성질
Clear, colorless to pale yellow, flammable liquid with a sharp, penetrating, nauseating fish-like odor. Odor threshold concentrations in water and air were 2 ppm (Buttery et al., 1988) and 21 ppbv (Leonardos et al., 1969), respectively. Detection odor threshold concentrations of 0.74 mg/m3 (2.3 ppmv) and 6 mg/m3 (1.9 ppmv) were experimentally determined by Katz and Talbert (1930) and Dravnieks (1974), respectively. Cometto-Mu?iz and Cain (1990) reported an average nasal pungency threshold concentration of 1,275 ppmv.출처
Pyridine was discovered by Anderson in coal tar in 1846 (Windholz et al 1983). It is found in tobacco smoke (Vohl and Eulenberg 1871; Lehmann 1909) and roasted coffee (Bertrand and Weisweiller 1913). Pyridine is found in wood oil and in the leaves and roots of Atropa belladonna (HSDB 1988), and is also a component of creosote oil (Krone et al 1986). In nature, pyridine and its derivatives are commonly found as components of alkaloids, vitamins, and coenzymes.용도
Pyridine is used directly in the denaturation of alcohol (ACGIH 1986; HSDB 1989; NSC 1978) and as a solvent in paint and rubber preparation (ACGIH 1986; HSDB 1989; NSC 1978) and in research laboratories for functions such as extracting plant hormones (Santodonato et al. 1985). Half of the pyridine produced today is used as an intermediate in making various insecticides and herbicides for agricultural applications (ACGIH 1986; Harper et al. 1985; Santodonato et al. 1985). Approximately 20% goes into the production of piperidine (Harper et al. 1985; Santodonato et al. 1985) which is commercially significant in the preparation of chemicals used in rubber vulcanization and agriculture (NSC 1978). Pyridine is also used as an intermediate in the preparation of drugs (antihistamines, steroids, sulfa-type and other antibacterial agents) dyes, water repellents, and polycarbonate resins (ACGIH 1986; Harper et al. 1985; NSC 1978; Santodonato et al. 1985). Pyridine is also approved by the Food and Drug Administration (FDA) for use as a flavoring agent in the preparation of foods (Harper et al. 1985; HSDB 1989) .제조 방법
Pyridine is produced either by isolation from natural sources such as coal, or through chemical synthesis (HSDB 1989). Pyridine is produced by the fractional distillation of coal-tar residues (HSDB 1989; NSC 1978; Santodonato et al. 1985) in which 1 ton of coal produces 0.07-0.21 pounds of pyridine bases of which 57% is pyridine (Santodonato et al, 1985). Synthetically produced pyridine is currently the more important source of pyridine for commercial uses (Santodonato et al. 1985). Small amounts of pyridine are synthesized from acetaldehyde, formaldehyde, and ammonia with a fluidized silica-alumina catalyst, followed by fractionation to isolate the pyridine (Harper et al. 1985; HSDB 1989; NSC 1978).Pyridine is produced from natural sources by Crowley Tar Products of Stow, Ohio, and Oklahoma City, Oklahoma (Harper et al. 1985; HSDB 1989; SRI 1986, 1987, 1988). Pyridine is synthetically produced by two companies, the Nepera Chemical Co. of Harriman, New York and the Reilly Tar and Chemical Corporation of Indianapolis, Indiana (Harper et al. 1985; SRI 1986, 1987, 1988).
정의
ChEBI: Pyridine is an azaarene comprising a benzene core in which one -CH group is replaced by a nitrogen atom. It is the parent compound of the class pyridines.The molecules have a hexagonal planar ring and are isoelectronic with benzene. Pyridine is an example of an aromatic heterocyclic compound, with the electrons in the carbon–carbon pi bonds and the lone pair of the nitrogen delocalized over the ring of atoms. The compound is extracted from coal tar and used as a solvent and as a raw material for organic synthesis.생산 방법
Pyridine is produced from the gases obtained by the coking of coal and by direct synthesis. The light-oil fraction of coal tar is treated with sulfuric acid to produce water-soluble pyridine salts and then the pyridine bases are recovered from the aqueous phase by sodium hydroxide or ammonia (Jori et al 1983). The majority of U.S. production is through synthetic means. This process uses a vapor-phase reaction of acetaldehyde, formaldehyde and ammonia, which yields a mixture of pyridine and 3-methylpyridine (Santodonato et al 1985). The product ratio depends on the relative amounts of acetaldehyde and formaldehyde. Added methanol increases the yield. The U.S. production of pyridine was estimated at 32 to 47 million pounds in 1975 (Reinhardt and Brittelli 1981). Pyridine is commercially available in technical, 2° and 1° grades, the latter two referring to their boiling ranges. Major impurities are higher boiling homologues, such as picolines, lutidines and collidines, which are mono-, di-, and trimethylpyridines (Santodonato et al 1985; Jori et al 1983).일반 설명
A clear colorless to light yellow liquid with a penetrating nauseating odor. Density 0.978 g / cm3. Flash point 68°F. Vapors are heavier than air. Toxic by ingestion and inhalation. Combustion produces toxic oxides of nitrogen.공기와 물의 반응
Highly flammable. Soluble in water.반응 프로필
Azabenzene is a base. Reacts exothermically with acids. During preparation of a complex of Azabenzene with chromium trioxide, an acid, the proportion of chromium trioxide was increased. Heating from this acid-base reaction led to an explosion and fire [MCA Case History 1284 1967]. A 0.1% solution of Azabenzene (or other tertiary amine) in maleic anhydride at 185°C gives an exothermic decomposition with rapid evolution of gas [Chem Eng. News 42(8); 41 1964]. Mixing Azabenzene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (96%), or propiolactone [NFPA 1991]. The combination of iodine, Azabenzene, sulfur trioxide, and formamide developed a gas over pressurization after several months. This arose from the slow formation of sulfuric acid from external water, or from dehydration of the formamide to hydrogen cyanide. Ethylene oxide and SO2 can react violently in Azabenzene solution with pressurization if ethylene oxide is in excess (Nolan, 1983, Case History 51).위험도
Flammable, dangerous fire risk, explosive limits in air 1.8–12.4%. Toxic by ingestion and inhalation. Skin irritant, liver and kidney damage. Questionable carcinogen.건강위험
The acute toxicity of pyridine is low. Inhalation causes irritation of the respiratory system and may affect the central nervous system, causing headache, nausea, vomiting, dizziness, and nervousness. Pyridine irritates the eyes and skin and is readily absorbed, leading to systemic effects. Ingestion of pyridine can result in liver and kidney damage. Pyridine causes olfactory fatigue, and its odor does not provide adequate warning of the presence of harmful concentrations.Pyridine has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. Chronic exposure to pyridine can result in damage to the liver, kidneys, and central nervous system.
인화성 및 폭발성
Pyridine is a highly flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance and "flash back." Pyridine vapor forms explosive mixtures with air at concentrations of 1.8 to 12.4% (by volume). Carbon dioxide or dry chemical extinguishers should be used for pyridine fires.공업 용도
Pyridine is a good solvent for a large number of compounds, both organic and inorganic (Windholz et al 1983). About 50% of pyridine used in the U.S. is for the production of agricultural chemicals, such as the herbicides paraquat, diquat and triclopyr and the insecticide chlorpyrifos. Other uses are in the production of piperidine; the manufacture of pharmaceuticals, such as steroids, vitamins and antihistamines; and as a solvent. Solvent uses are found in both the pharmaceutical and polycarbonate resin industries. It is particularly useful as a solvent in processes where HC1 is evolved (Santodonato et al 1985). Minor uses for pyridine are for the denaturation of alcohol and antifreeze mixtures, as a dyeing assistant in textiles and as a flavoring agent (Jori et al 1983; Furia 1968; HSDB 1988).색상 색인 번호
Pyridine (unsubstituted pyridine) and its derivative (substituted pyridines) are widely used in chemistry. Pyridine is a solvent used for many organic compounds and anhydrous metallic salt chemicals. Contained in Karl Fischer reagent, it induced contact dermatitis in a laboratory technician. No cross-sensitivity is observed between those different substances.Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion, skin contact, intravenous, and subcutaneous routes. Mildly toxic by inhalation. A skin and severe eye irritant. Mutation data reported. Can cause central nervous system depression, gastrointestinal upset, and liver and kidney damage. A flammable liquid and dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard in the form of vapor when exposed to flame or spark. Reacts violently with chlorosulfonic acid, chromium trioxide, dinitrogen tetraoxide, HNO3, oleum, perchromates, ppropiolactone, AgClO4, H2SO4. Incandescent reaction with fluorine. Reacts to form pyrophoric or explosive products with bromine trifluoride, trifluoromethyl hypofluorite. Mixtures with formamide + iodine + sulfur trioxide are storage hazards, releasing carbon dioxide and sulfuric acid. Incompatible with oxidizing materials. Reacts with maleic anhydride (above 150°C) evolving carbon dioxide. To fight fire, use alcohol foam. When heated to decomposition it emits highly toxic fumes of NOx.잠재적 노출
Pyridine is used as a solvent in the chemical industry and as a denaturant for ethyl alco- hol; as an intermediate in the production of pesticides; in pharmaceuticals; in the manufacture of paints, explosives, dyestuffs, rubber, vitamins, sulfa drugs; and disinfectants.Carcinogenicity
Pyridine was not carcinogenic in several chronic subcutaneous studies.F344 rats were given pyridine orally in drinking water at doses of 0, 7, 14, or 33 mg/kg for 2 years. The top dose produced a decrease in body weights and water consumption. Increased renal tubular adenoma or carcinoma and tubular hyperplasia were observed in males at 33 mg/kg. Increased mononuclear cell leukemia was observed in females at 14 and 33 mg/kg, which was considered equivocal in terms of the relationship to pyridine exposure, since this is a common finding in this strain of rat. Concentration-related nonneoplastic change in the liver was seen at 33 mg/kg. Male Wistar rats were similarly treated with doses of 0, 8, 17, or 36 mg/kg for 2 years. Decreased survival and body weights were seen at 17 and 36 mg/kg. Increased testicular cell adenomas were seen at 36 mg/kg. No changes in survival or neoplasm rates in other tissues, including the kidney, were reported although increased nephropathy and hepatic centrilobular degeneration/necrosis was observed in some pyridine- treated rats.
환경귀착
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.31 g/g which is 58.7% of the ThOD value of 2.23 g/g. A Nocardia sp. isolated from soil was capable of transforming pyridine, in the presence of semicarbazide, into an intermediate product identified as succinic acid semialdehyde (Shukla and Kaul, 1986). 1,4-Dihydropyridine, glutaric dialdehyde, glutaric acid semialdehyde, and glutaric acid were identified as intermediate products when pyridine was degraded by Nocardia strain Z1 (Watson and Cain, 1975).Photolytic. Irradiation of an aqueous solution at 50 °C for 24 h resulted in a 23.06% yield of carbon dioxide (Knoevenagel and Himmelreich, 1976).
Chemical/Physical. The gas-phase reaction of ozone with pyridine in synthetic air at 23 °C yielded a nitrated salt having the formula: [C6H5NH]+NO3 - (Atkinson et al., 1987). Ozonation of pyridine in aqueous solutions at 25 °C was studied with and without the addition of tert-butyl alcohol (20 mM) as a radical scavenger. With tert-butyl alcohol, ozonation of pyridine yielded mainly pyridine N-oxide (80% yield), which was very stable towards ozone. Without tert-butyl alcohol, the heterocyclic ring is rapidly cleaved forming ammonia, nitrate, and the amidic compound N-formyl oxamic acid (Andreozzi et al., 1991).
저장
Pyridine should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.운송 방법
UN1992 Flammable liquids, toxic, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous mate- rials, Technical Name Required.비 호환성
Violent reaction with strong oxidizers; strong acids; chlorosulfonic acid; maleic anhydride; oleum iodine.폐기물 처리
Controlled incineration whereby nitrogen oxides are removed from the effluent gas by scrubber, catalytic or thermal devices .피리딘 준비 용품 및 원자재
원자재
준비 용품
4-BROMO-TETRAHYDROPYRAN
N-PHENYLISONICOTINAMIDE
2-AMINO-6-CHLORO-3,5-DICYANOPYRIDINE
피리디늄 파라-톨루엔술폰산
이염화파라콰트
1-CHLORO-2-METHYLPROPYL CHLOROFORMATE
1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one
2-시아노피라진
7-ACETOXYCOUMARIN
Phenylcarbamic acid propyl ester
2-Amino-4-methyl-5-acetylthiazole
5-ACETAMIDONICOTINIC ACID
Benzyl 2-chloroacetate
trans-Ferulic acid
2-AMINO-4-METHYL-QUINOLINE-3-CARBONITRILE
3-(Trifluoromethyl)pyrazole
3-(트리플루오로메톡시)신남산
1-페나실피리디니움 브로마이드
(4-FLUORO-BENZYL)-METHYL-AMINE
5-METHYLPICOLINIC ACID
Indigosol Green Blue IBC
2,4-MESITYLENEDISULFONYL DICHLORIDE
17beta-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one
디에틸렌글리콜노르말부틸에테르
카보닐산알릴메틸에스테르
3,5-DIMETHOXYCINNAMIC ACID
4-Acetamido-2-chloropyridine
4-플루오로신나믹 산
히드로코르티손 아세트산
Methyl 2-Fluoroisonicotinate
2-ACETYL-5-CYANOTHIOPHENE
5-BROMO-2-FLUOROCINNAMIC ACID
4-NITROISOPHTHALIC ACID
시린잘데하이드
2,4,5,6-TETRAMETHYLBENZENEDISULFONYL DICHLORIDE
Pyridine-3-sulfonyl chloride hydrochloride
3-Methoxycinnamic acid
butyl N-phenylcarbamate
3-(3-METHYL-2-THIENYL)ACRYLIC ACID
Vat Grey M
피리딘 공급 업체
글로벌( 899)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hubei Langyou International Trading Co., Ltd | +8618874586545 |
linda@xrdchem.cn | China | 197 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 142 | 55 |
피리딘 관련 검색:
3,4,7,8-사메틸-1,10-페난트롤린 하르민 피리딘 알릴 알코올 메틸알콜 4-피리딘붕소산 2-아미노-5-나이트로피리딘 2-아미노-3-히드록시피리딘 네오쿠프로인
2-Pyridinecarboxaldehyde
Pyridine hydrochloride
1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one
Pyridine sulfur trioxide
SODIUM GLUCOHEPTONATE
5-METHYL-1,10-PHENANTHROLINE
4,7-DIHYDROXY-1,10-PHENANTHROLINE
Bathophenanthroline
4,7-Dimethyl-1,10-phenanthroline