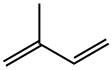
이소프렌(2-메틸-1,3-부타디엔)
|
|
이소프렌(2-메틸-1,3-부타디엔) 속성
- 녹는점
- 323-329 °C(lit.)
- 끓는 점
- 34 °C(lit.)
- 밀도
- 0.681 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.35 (vs air)
- 증기압
- 8.82 psi ( 20 °C)
- 굴절률
- n
20/D 1.422(lit.)
- 인화점
- −65 °F
- 저장 조건
- Store at <= 20°C.
- 용해도
- 0.7g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- >14 (Schwarzenbach et al., 1993)
- 색상
- 투명한 무색~매우 연한 노란색
- 냄새
- 석유 냄새
- Odor Threshold
- 0.048ppm
- 폭발한계
- 1-9.7%(V)
- 수용성
- 0.07g/100mL
- 어는점
- -145.96℃
- 최대 파장(λmax)
- 231nm(neat)(lit.)
- Merck
- 14,5201
- BRN
- 969158
- Henry's Law Constant
- (x 10-2 atm?m3/mol): 3.45 at 18 °C (dynamic stripping cell-MS, Karl et al., 2003)
- Dielectric constant
- 2.1(25℃)
- 안정성
- Stability Extremely flammable. Readily forms explosive mixtures with air. Note low flash point, low boiling point, high vapour pressure. Unstable - prone to spontaneous polymerization. May contain a polymerization inhibitor. Incompatible with strong oxidizing agents.
- LogP
- 2.42 at 20℃
- CAS 데이터베이스
- 78-79-5(CAS DataBase Reference)
- IARC
- 2B (Vol. 60, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F+,T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-12-52/53-68-51/53 | ||
| 안전지침서 | 53-45-61-36/37-16 | ||
| 유엔번호(UN No.) | UN 1218 3/PG 1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | NT4037000 | ||
| 자연 발화 온도 | 428 °F | ||
| TSCA | Yes | ||
| HS 번호 | 2901 24 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 78-79-5(Hazardous Substances Data) | ||
| 독성 | LD50 for mice: 144 mg isoprene vapors/l air (Gostinskii) | ||
| 기존화학 물질 | KE-23526 | ||
| 유해화학물질 필터링 | 2019-1-932 | ||
| 중점관리물질 필터링 | 별표1-22 | ||
| 사고대비 물질 필터링 | 78 | ||
| 함량 및 규제정보 | 물질구분: 사고대비물질; 혼합물(제품)함량정보: 이소프렌 및 이를 25% 이상 함유한 혼합물 |
이소프렌(2-메틸-1,3-부타디엔) C화학적 특성, 용도, 생산
용도
메트포르민(metformin)은 바이구아니드계(biguanides) 경구용 당뇨병치료제이다. 혈당개선효과가 있으나 심혈관질환을 예방하는 효과는 증거가 아직 제한적이다. 작용기전의 하나로 간에서 AMP-activated protein kinase (AMPK)를 활성화함으로써 포도당신생합성을 막고, 세포에 포도당이 흡수되는 것을 촉진하고, 대사증후군을 억제한다. 이 약물은 2형 당뇨병 치료에 1차 약물로 사용되며, 특히 정상 신기능을 가지는 과체중 혹은 비만 환자에게 사용된다. 하지만 임신한 여성에게는 안전성의 문제로 사용이 제한된다. 메트포르민은 다낭성 난소 증후군치료에도 사용되며 다른 적응증도 연구중이다. 메트포르민은 간에서 포도당의 생성을 억제한다.용도
아 다만 탄 은 매우 중요한 화학 중간체 입니다.화학적 성질
Isoprene (2-methyl-l,3-butadiene) is a colorless, volatile, flammable liquid with specific gravity 0.6758. It is highly reactive, usually occurs as its dimer, and unless inhibited undergoes explosive polymerization. Isoprene naturally occurs in the environment as emissions from vegetation. It may be released to the environment as emissions during wood pulping, biomass combustion, and rubber abrasion; through tobacco smoke, gasoline, turbine, and automobile exhaust. In tobacco smoke, isoprene has been determined to be the precursor of a number of polycyclic aromatics, as demonstrated by thermal condensations in the range of 450–700℃.물리적 성질
Colorless, volatile, extremely flammable liquid with an petroleum-like odor. An odor threshold concentration of 48 ppbV was reported by Nagata and Takeuchi (1990).용도
Isoprene occurs in nature and it is produced by many plants. Its polymers are the main component of natural rubber. The most important application of isoprene is to manufacture polymers and copolymers. Polyisoprene, a synthetic rubber made from isoprene, is used in a wide variety of rubber applications including medical equipment, baby bottle teats/nipples, toys, shoe soles, tires, elastic films, threads for golf balls or textiles, adhesives, paints, and coatings. Copolymer butyl rubber, made from isobutene with a small amount of isoprene, has excellent impermeability to gases and is used in inner tubes. Another copolymer styrene-isoprene rubber is used in pressure sensitive adhesives. Isoprene is also used as a chemical intermediate.제조 방법
Isoprene is obtained from propylene by the followin,g route: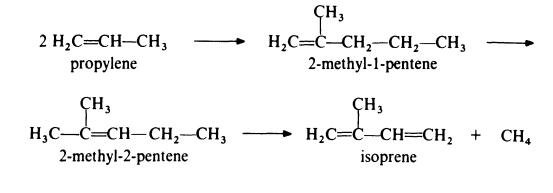
In the first step, propylene is dimerized to 2-methyl-l-pentene by passage over a catalyst of tri-n-propylaluminium at about 200??C and 20 MPa (200 atmospheres). This product is then isomerized to 2-methyl-2-pentene by heating at 150-300??C in the presence of a silica-alumina catalyst. The final step in the process is the pyrolysis of the olefin to isoprene at 650-800??C in the presence of a free radical initiator such as hydrogen bromide. The isomerization step is necessary because pyrolysis of 2-methyl-l-pentene gives much poorer yields of isoprene than pyrolysis of 2-methyl-2-pentene.
생산 방법
Rubber results from the polymerization of isoprene to form polyisoprene. The resultingstructure dictates the properties of the rubber. Natural rubber has a cis 1,4 structure.This means that the carbon atoms that form the chainattach to the same side ofthe chain at the 1 and 4 positions. The cisstructure gives rubber its elasticity. Polyisoprene alsoexists in a trans 1,3 configuration. In the trans configuration, the addition takes place onopposite sides of the carbon chain.Natural rubber occurs in a colloidal milky suspension called latex, which is obtained fromnumerous plants. The most important of these is the para rubber tree, Hevea brasiliensis. Naturalrubber is harvested by cutting a v-shape incision into a plant and allowing latex to drain intoa container containing a preservative. About 50mL of latex is obtained on a daily basis. Latexis transported to collection stations where it is processed for shipment. Processing can includepreservation, coagulation, and concentrating before being sent to rubber factories.
정의
ChEBI: A hemiterpene with the formula CH22C(CH3)CH2CH2; the monomer of natural rubber and a common structure motif to the isoprenoids, a large class of other naturally occurr ng compounds.일반 설명
A clear colorless liquid with a petroleum-like odor. Density 5.7 lb / gal. Flash point -65°F. Boiling point 93°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Less dense than water and insoluble in water. Vapors heavier than air.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
ISOPRENE may react vigorously with strong oxidizing agents. May react exothemically with reducing agents to release hydrogen gas. May undergo exothermic addition polymerization in the presence of various catalysts (such as acids) or initiators. Undergoes autoxidation upon exposure to the air to form explosive peroxides. Mixing isoprene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (90%) [NFPA 1991].위험도
Highly flammable, dangerous fire and explosion risk. Irritant. Possible carcinogen.건강위험
Vapor produces no effects other than slight irritation of the eyes and upper respiratory tract. Liquid may irritate eyes; like gasoline.Carcinogenicity
Isoprene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.환경귀착
At 25 ℃, isoprene has a high vapor pressure of 733 hPa, a low water solubility of 642 mg l-1, and a Henry’s law constant of 7781 Pam3 mol-1. Isoprene’s log Kow is 2.42 while its log Koc is 1.83. Isoprene’s vapor density relative to air is 2.4. Because of its high vapor pressure at ambient temperature, isoprene will partition largely into the atmosphere, with negligible amounts partitioning to soil and water. Due to a short half-life in air (0.5 h by reaction with nitric oxide, 1.2–4 h by reaction with hydroxyl radicals, and 19 h by reaction with ozone), wet deposition of isoprene from air is not expected to play a significant role in its atmospheric fate. Although laboratory testing demonstrates that isoprene has the potential to biodegrade, microbial metabolism is unlikely to contribute significantly to the removal of isoprene from the environment due to rapid volatilization from terrestrial and aquatic media. Isoprene has a low bioaccumulation potential and is not expected to bioaccumulate.Purification Methods
Reflux it with sodium then distil it from sodium or NaBH4 under nitrogen, and pass it through a column containing KOH, CaSO4 and silica gel. tert-Butylcatechol (0.02% w/w) is added, and the isoprene is stored in this way until redistilled before use. The inhibitor (tert-butylcatechol) in isoprene can be removed by several washings with dilute NaOH and water. The isoprene is then dried over CaH2, distilled under nitrogen at atmospheric pressure, and the fraction distilling at 32o is collected. Store it under nitrogen at -15o. [Beilstein 1 H 252, 1 IV 1001.]이소프렌(2-메틸-1,3-부타디엔) 준비 용품 및 원자재
원자재
준비 용품
2,2-DIMETHYLCYCLOPENTANONE
3-METHYL-2,5-DIHYDROTHIOPHENE-1,1-DIOXIDE
메틸(2-)-2-헵텐-6-온
메틸(3-)-2-부텐-1-올
이소부틸렌-이소프렌 공중합체
아밀렌
p-메틸 이오논
사이클로펜텐
TERPINEOL
클로로-3-메틸-2-부텐
Methyl tetrahydrophthalic anhydride
메틸이소프로필케톤
3,7-dimethyl-1,7-octadien-3-ol
2,6-Dimethyl-1,7-octadien-3-ol
3,6-dihydro-4-methyl-2-(2-methylpropyl)-2H-pyran
DECANE,3,8-DIMETHYL-
이소프렌(2-메틸-1,3-부타디엔) 공급 업체
글로벌( 271)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 |
1057@dideu.com | China | 3537 | 58 |
| Shandong Luning Pharmaceutical Co., Ltd. | 0546-6491488 +8613305469775 |
China | 40 | 58 |