| Company Name: |
|
| Tel: |
|
| Email: |
manish@purechagroup.com |
| Products Intro: |
Cas:103-63-9
ProductName:2-Phenylethyl bromide
Purity: 98% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
|
| Tel: |
|
| Email: |
info@sontaraorgano.com |
| Products Intro: |
Cas:103-63-9
ProductName:2-Phenylethyl bromide
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
|
| Tel: |
|
| Email: |
bhavikachemicals@yahoo.com |
| Products Intro: |
Cas:103-63-9
ProductName:2-Phenylethyl bromide
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg
|
- Ethyl Bromide
-

- $9.00 / 1KG
-
2023-03-06
- CAS:74-96-4
- Min. Order: 1KG
- Purity: 99.8%
- Supply Ability: 100tons
- Bromoethane, Ethyl Bromide
-

- $200.00 / 1Kg/Bag
-
2022-12-15
- CAS:74-96-4
- Min. Order: 1Kg/Bag
- Purity: Above 99%
- Supply Ability: 5000kg/month
|
| | Phenyl Ethyl Bromide Basic information |
| Product Name: | Phenyl Ethyl Bromide | | Synonyms: | PHENYL ETHYL BROMIDE;(2-bromoethyl)-benzen;.beta.-Phenylethylbromide;2-phenethylbromide;2-Phenyl-1-bromoethane;Benzene, (2-bromoethyl)-;Benzene,(2-bromoethyl)-;benzene,2-bromoethyl- | | CAS: | 103-63-9 | | MF: | C8H9Br | | MW: | 185.06 | | EINECS: | 203-130-8 | | Product Categories: | fine chemicals;103-63-9 | | Mol File: | 103-63-9.mol | 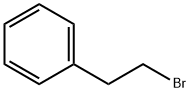 |
| | Phenyl Ethyl Bromide Chemical Properties |
| Melting point | -56 °C | | Boiling point | 220-221 °C(lit.) | | density | 1.355 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.556(lit.) | | Fp | 193 °F | | storage temp. | 2-8°C | | solubility | 0.039g/l | | form | Liquid | | color | Clear colorless to pale yellow | | Water Solubility | INSOLUBLE | | BRN | 507487 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChIKey | WMPPDTMATNBGJN-UHFFFAOYSA-N | | CAS DataBase Reference | 103-63-9(CAS DataBase Reference) | | NIST Chemistry Reference | 1-Bromo-2-phenylethane(103-63-9) | | EPA Substance Registry System | Benzene, (2-bromoethyl)- (103-63-9) |
| | Phenyl Ethyl Bromide Usage And Synthesis |
| Description | Phenethylbromide is an analytical reference material that is structurally categorized as an organobromide. It is used in the synthesis of a variety of compounds, including fentanyl. Phenethylbromide is combined with 4-piperidinone to produce N-phenethyl-4-piperidone, which, as a precursor in the synthesis of fentanyl, is scheduled as a List I chemical in the United States. Phenethylbromide may be found as in impurity in samples of fentanyl produced using this pathway. | | Chemical Properties | colourless to yellow liquid. It is miscible with ether and benzene, but insoluble in water. | | Uses | (2-Bromoethyl)benzene is used in the preparation of phenelzine by reacting with hydrazine. It is also used as a starting material to prepare various beta-phenethyl derivatives, active pharmaceutical ingredients, and fragrances. It finds application as a flame retardant. | | Uses | (2-Bromoethyl)benzene is an important
starting material for the production of various
b-phenethyl derivatives, pharmaceuticals, fragrances, and other fine chemicals. | | Preparation | (2-Bromoethyl)benzene is synthesized by the reaction of phenethyl alcohol with hydrogen bromide.
Reaction: The phenethyl alcohol was heated to 110°C, hydrogen bromide was slowly introduced, and the reaction was refluxed. The reaction was completed, cooled, washed with water, 10% sodium carbonate solution, and water in turn. After drying with anhydrous potassium carbonate, fractional distillation under reduced pressure was carried out to collect fractions at 97-99°C (2.0 kPa) with a yield of over 90%. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 29, p. 2317, 1964 DOI: 10.1021/jo01031a051
Synthetic Communications, 20, p. 2349, 1990 DOI: 10.1080/00397919008053179 | | Safety | Store at 2-8° C in a tightly closed container in a dry, well-ventilated place. Containers that are opened must be carefully resealed and kept upright to prevent leakage. (2-Bromoethyl)benzene is incompatible with strong oxidizing agents. This chemical is harmful if swallowed and causes severe eye irritation. | | Synthesis | Phenethylbromide is commonly synthesized through the addition of HBr to styrene via a free-radical reaction. This process is typically carried out in a glasslined reactor by introducing gaseous HBr into a solution of styrene in an inert solvent that contains a free-radical initiator. The reaction, which is fast and releases a moderate amount of heat, can be managed by introducing HBr at a rate that matches its consumption. Once all the styrene has been converted, any remaining excess HBr can be reclaimed using different methods before the solvent and product are recovered through distillation. | | Purification Methods | Wash the bromide with conc H2SO4, water, aqueous 10% Na2CO3 and water again, then dry it with CaCl2 and fractionally distil it just before use. [Beilstein 5 IV 907.] | | References | 1. Shukla, P.; Sharma, A.; Pallavi, B.; Cheng, C. H. Nickel-catalyzed reductive Heck type coupling of saturated alkyl halides with acrylates and oxabenzonorbornadiene. Tetrahedron 2015, 71 (15), 2260-2266. DOI:10.1016/J.TET.2015.02.067
2. Huang, Y. B.; Yan, L.; Chen, M. Y.; Guo, Q. X.; Fu, Y. Selective hydrogenolysis of phenols and phenyl ethers to arenes through direct C-O cleavage over ruthenium-tungsten bifunctional catalysts. Green Chem. 2015, 17 (5), 3010-3017. DOI:10.1039/C5GC00326A
3. Justice, D.E.A.D.o. Control of a chemical precursor used in the illicit manufacture of fentanyl as a List I chemical. Final rule. Federal Register 73(144), 43355-43357 (2008). PMID: 18949877 |
| | Phenyl Ethyl Bromide Preparation Products And Raw materials |
|