| | NITROUS OXIDE Basic information |
| Product Name: | NITROUS OXIDE | | Synonyms: | hemioxyded’azote;Hyponitrous acid anhydride;hyponitrousacidanhydride;Nitral;Nitrogen oxide (N2O);nitrogenhypoxide;nitrogenoxide(n2o);nitrousoxide,compressed | | CAS: | 10024-97-2 | | MF: | N2O | | MW: | 44.01 | | EINECS: | 233-032-0 | | Product Categories: | refrigerants;Inorganics | | Mol File: | 10024-97-2.mol | 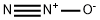 |
| | NITROUS OXIDE Chemical Properties |
| Melting point | −91 °C(lit.) | | Boiling point | −88 °C(lit.) | | density | 1.23 g/cm3 (-89 ºC) | | vapor density | 1.53 (15 °C, vs air) | | vapor pressure | 51.7 mm Hg ( 21 °C) | | FEMA | 2779 | NITROUS OXIDE | | refractive index | 1.380 | | solubility | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 1.5 volumes of water. | | form | colorless gas | | pka | -16.68±0.53(Predicted) | | color | colorless | | Odor | odorless | | Water Solubility | slightly soluble H2O; soluble alcohol, ether, conc H2SO4 [HAW93] | | Merck | 13,6687 | | BRN | 8137358 | | Dielectric constant | 1.6(0℃) | | Stability: | Oxidant, strongly supports combustion. May react violently with some materials. Thermal decomposition yields toxic products. Incompatible with aluminium, boron oxides, hydrazine, strong reducing agents. | | LogP | 0.43 | | CAS DataBase Reference | 10024-97-2(CAS DataBase Reference) | | EPA Substance Registry System | Nitrous oxide (10024-97-2) |
| Hazard Codes | O | | Risk Statements | 8 | | Safety Statements | 38 | | RIDADR | UN 1070 2.2 | | OEB | A | | OEL | TWA: 25 ppm (46 mg/m3) (TWA: over the time exposed) [*Note: REL for exposure to waste anesthetic gas.] | | WGK Germany | 1 | | RTECS | QX1350000 | | F | 4.5-31 | | DOT Classification | 2.2 (Nonflammable gas) | | HazardClass | 2.2 | | Hazardous Substances Data | 10024-97-2(Hazardous Substances Data) | | Toxicity | Because of its analgesic effects and the moderate loss of
inhibitions, it has been frequently abused. Such chronic problems may cause long-term toxicity not seen with appropriate
use, including possible effects on the male reproductive system. |
| | NITROUS OXIDE Usage And Synthesis |
| Description | dinitrogen monoxide’s (N2O)
common name is nitrous oxide.Nitrous oxide is a colorless, nonfl ammable,
nontoxic gas with a slightly sweet odor and taste. Nitrous oxide is produced by
the thermal decomposition of ammonium nitrate at approximately 240°C: NH4NO3(g) →
N2O(g) + 2H2O(g).Nitrous oxide is an important greenhouse gas.
Its atmospheric residence time is 120 years. A molecule of N2O has 310 times the potential
for absorbing heat compared to a molecule of CO2. Nitrous oxide is stable and unreactive on
the earth’s surface, but it can be transported to the stratosphere where it absorbs energy and is
converted into reactive forms of nitrogen such as nitric oxide and the nitrate radical contributing
to ozone destruction. | | Chemical Properties | Nitrous oxide is a nonflammable, colorless and odorless, sweettasting
gas. It is usually handled as a compressed gas, stored in metal
cylinders. | | Chemical Properties | A colorless gas without appreciable odor or a slightly sweetish odor and taste. One L at 0°C and at a pressure of 760 mm of mercury weighs about 1.97 g. One volume dissolves in about 1.4 volumes of water at 20°C and at a pressure of 760 mm of mercury. It is freely soluble in alcohol and soluble in ether and in oils. It is prepared by thermal decomposition of ammonium nitrate. | | Chemical Properties | Colourless gas with sweetish odour | | Chemical Properties | Nitrous oxide has a slight sweetish, odor and taste. This gas is also reported as without appreciable odor. At 0°C and a
pressure of 760 mm of mercury, 1 L weighs about 1.97 g. | | Chemical Properties | Nitrous oxide is a colorless gas. Slightly sweet
odor. Shipped as a liquefied compressed gas. | | Physical properties | Colorless gas with faint sweet odor and taste; heavier than air, density in air 1.53 (air=1); gas density 1.977 g/L at 0°C; noncombustible gas; supports combustion; liquefies to a colorless liquid at -88.5°C; liquid density 1.226 g/mL at -89°C; freezes to a cubic crystalline solid at -90.8°C; dipole moment 0.166 ; critical temperature 36.5°C; critical pressure 71.7 atm; solubility in water: 130 mL gas dissolves in 100mL water at 0°C and 56.7 mL in 100 mL water at 25°C; soluble in alcohol, ether and sulfuric acid. | | History | nitrous oxide was prepared in 1772 by Joseph Priestley (1733 1804) . Priestley called nitric oxide nitrous air, nitrogen dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper: 3Cu(s) + 8HNO3(aq) 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).He prepared nitrous oxide by reducing nitric oxide using iron: 2NO(g) + H2O(l) + Fe(s) N2O(g) + Fe(OH)2(aq).For example, the year of discovery for nitrous oxide ranges between 1772 and 1793. Humphrey Davy (1778 1829) examined the physiological effects of nitrous oxide and in 1799 wrote Researches Chemical and Philosophical, Chiefly Concerning Nitrous Oxide. | | Uses | Nitrous oxide is called laughing gas and has been used as a recreational inhalant, anesthetic, oxidizer, and propellant. Nitrous oxide is widely used as an anesthetic in dental surgery, which accounts for approximately 90% of its use. It is used by the dairy industry as a foaming agent for canned whipping creams.
The gas is used as an anesthetic, especially in dentistry and minor surgery. It produces mild hysteria and laughter preceding the anesthetic effect, for which reason it also is called “laughing gas.” It is used as an aerosol propellant, an aerating agent for whipped cream, and an oxidizing agent at high temperatures. Nitrous oxide also is used in the preparation of nitrites and as a flame gas in flame atomic absorption spectrometry of metals. | | Uses | Nitrous oxide was discovered by Priestley. It is found in the atmosphere in trace concentrations. The gas is used as an anesthetic, especially in dentistry and minor surgery. It produces mild hysteria and laughter preceding the anesthetic effect, for which reason it also is called “laughing gas.” It is used as an aerosol propellant, an aerating agent for whipped cream, and an oxidizing agent at high temperatures. Nitrous oxide also is used in the preparation of nitrites and as a flame gas in flame atomic absorption spectrometry of metals. | | Uses | Nitrous oxide is still commonly used in combination with a volatile agent
to maintain anaesthesia. However, there is growing concern regarding its
toxic effects and cost. Consequently, medical air in combination with oxygen
is now being used increasingly during anaesthesia. | | Uses | Nitrous Oxide is a noncombustible gas used as a propellant in certain
dairy and vegetable fat whipped toppings contained in pressurized
containers. | | Uses | Nitrous oxide is used in the productionof nitrites, in rocket fuel, as an inhalationanesthesia and analgesic agent. | | Preparation | Prepared by thermal decomposition of ammonium nitrate
NH4NO3 → N2O↑ + 2H2O | | Production Methods | Prepared (1) by reaction of silver hyponitrite Ag2N2O2 and hydrogen chloride in anhydrous ether, an evaporation of the resulting solution, (2) by reaction of hydroxylamine H2NOH plus nitrous acid HONO. | | Definition | A colorless gas with a faintly
sweet odor and taste. It is appreciably soluble
in water (1.3 volumes in 1 volume of
water at 0°C) but more soluble in ethanol.
It is prepared commercially by the careful
heating of ammonium nitrate:
NH4NO3(s) = N2O(g) + 2H2O(g)
Dinitrogen oxide is fairly easily decomposed
on heating to temperatures above
520°C, giving nitrogen and oxygen. The
gas is used as a mild anesthetic in medicine
and dentistry, being marketed in small steel
cylinders. It is sometimes called laughing
gas because it induces a feeling of elation
when inhaled. | | Definition | ChEBI: A nitrogen oxide consisting of linear unsymmetrical molecules with formula N2O. While it is the most used gaseous anaesthetic in the world, its major commercial use, due to its solubility under pressure in vegetable fats combined with
ts non-toxicity in low concentrations, is as an aerosol spray propellant and aerating agent for canisters of 'whipped' cream. | | Production Methods | Nitrous oxide is prepared by heating ammonium nitrate to about
170°C. This reaction also forms water. | | Biological Functions | N2O (commonly called laughing gas) produces its anesthetic
effect without decreasing blood pressure or
cardiac output. Although it directly depresses the myocardium,
cardiac depression is offset by an N2O–
mediated sympathetic stimulation. Likewise, respiration
is maintained.Tidal volume falls, but minute ventilation
is supported by a centrally mediated increase in respiratory
rate. However, since the respiratory depressant
effect of N2O are synergistic with drugs such as the opioids opioids
and benzodiazepines, N2O should not be considered
benign.
Deep levels of anesthesia are unattainable, even
when using the highest practical concentrations of N2O
(N2O 60–80% with oxygen 40–20%). Although unconsciousness
occurs at these inspired levels, patients exhibit
signs of CNS excitation, such as physical struggling
and vomiting. If the airway is unprotected, vomiting
may lead to aspiration pneumonitis, since the protective
reflexes of the airway are depressed.
On the other hand, lower inspired concentrations
(25–40%) of N2O produce CNS depression without excitatory
phenomena and are more safely used clinically.
CNS properties of low inspired tension of N2O include
periods of waxing and waning consciousness, amnesia,
and extraordinarily effective analgesia. N2O 25% produces
the gas’s maximum analgesic effect.With this concentration,
responses to painful surgical manipulations
are blocked as effectively as they would be with a therapeutic
dose of morphine. Such low inspired concentrations
of N2O are used in dentistry and occasionally for selected
painful surgical procedures (i.e., to relieve the pain
of labor). Since the tissue solubility of N2O is low, the
CNS effects are rapid in onset, and recovery is prompt
when the patient is returned to room air or oxygen.
The most common use of N2O is in combination with
the more potent volatile anesthetics. It decreases the
dosage requirement for the other anesthetics, thus lowering
their cardiovascular and respiratory toxicities. For example,
an appropriate anesthetic maintenance tension
for N2O and halothane would be N2O 40% and
halothane 0.5%.With this combination in a healthy patient,
anesthesia is adequate for major surgery, and the
dose-dependent cardiac effects of halothane are reduced. | | General Description | NITROUS OXIDE is a colorless, sweet-tasting gas. NITROUS OXIDE is also known as "laughing gas". Continued breathing of the vapors may impair the decision making process. NITROUS OXIDE is noncombustible but NITROUS OXIDE will accelerate the burning of combustible material in a fire. NITROUS OXIDE is soluble in water. Its vapors are heavier than air. Exposure of the container to prolonged heat or fire can cause NITROUS OXIDE to rupture violently and rocket. NITROUS OXIDE is used as an anesthetic, in pressure packaging, and to manufacture other chemicals. | | General Description | Nitrous oxide is a gas at room temperature and is supplied asa liquid under pressure in metal cylinders. Nitrous oxide is a“dissociative anesthetic” and causes slight euphoria and hallucinations. | | Reactivity Profile | NITROUS OXIDE is an oxidizing agent. Nonflammable but supports combustion. Can explode at high temperature (after vaporization). Vapors can undergo a violent reaction with aluminum, boron, hydrazine, lithium hydride, phenyllithium, phosphine, sodium, tungsten carbide [Bretherick, 5th ed., 1995, p. 1686]. Contact of the cold liquefied gas with water may result in vigorous or violent boiling. If the water is hot, a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquefied gas contacts water in a closed container [Handling Chemicals Safely 1980]. | | Hazard | Supports combustion, can form explosive
mixture with air. Narcotic in high concentration.
Central nervous system impairment, hematologic
effects, and embryo/fetal damage. Questionable
carcinogen. | | Health Hazard | Inhalation causes intense analgesia; concentrations of over 40-60% cause loss of consciousness preceded by hysteria. Contact of liquid with eyes or skin causes frostbite burn. | | Health Hazard | Toxicity and irritant effects of nitrous oxidein humans are very low. It is an anesthetic.Inhalation of this gas at high concentrationscan produce depression of the central nervous system, decrease in body temperature,and fall in blood pressure. The LC50 valueof a 4-hour exposure in mice is in the rangeof 600 ppm. | | Fire Hazard | Behavior in Fire: Will support combustion, and may increase intensity of fire. Containers may explode when heated. | | Pharmaceutical Applications | Nitrous oxide and other compressed gases such as carbon dioxide
and nitrogen are used as propellants for topical pharmaceutical
aerosols. They are also used in other aerosol products that work
satisfactorily with the coarse aerosol spray that is produced with
compressed gases, e.g. furniture polish and window cleaner.
The advantages of compressed gases as aerosol propellants are
that they are less expensive, of low toxicity, and practically odorless
and tasteless. In contrast to liquefied gases, their pressures change
relatively little with temperature. However, there is no reservoir of
propellant in the aerosol, and as a result the pressure decreases as
the product is used, changing the spray characteristics.
Misuse of a product by the consumer, such as using a product
inverted, results in the discharge of the vapor phase instead of the
liquid phase. Since most of the propellant is contained in the vapor
phase, some of the propellant will be lost and the spray
characteristics will be altered. Additionally, the sprays produced
using compressed gases are very wet. However, recent developments
in valve technology have reduced the risk of misuse by making
available valves which will spray only the product (not propellant)
regardless of the position of the container. Additionally, barrier
systems will also prevent loss of propellant, and have found
increased use with this propellant.
Therapeutically, nitrous oxide is best known as an anesthetic
administered by inhalation. When used as an anesthetic it has
strong analgesic properties but produces little muscle relaxation.
Nitrous oxide is always administered in conjunction with oxygen
since on its own it is hypoxic. | | Materials Uses | Nitrous oxide is noncorrosive and may therefore
be used with any of the common, commercially
available metals. Because of its oxidizing ac�tion, however, all equipment being prepared to
handle nitrous oxide, particularly at high pres�sures, must be free of oil, grease, and other
readily combustible materials. Nitrous oxide
may cause swelling ofsome elastomers. | | Clinical Use | The low potency of nitrous oxide (MAC= 104%) precludes it from being used alone for surgical anesthesia.To use it as the sole anesthetic agent the patient wouldhave to breathe in pure N2Oto the exclusion of oxygen. Thissituation would obviously cause hypoxia and potentially leadto death. Nitrous oxide can inactivate methionine synthase, aB12-dependent enzyme necessary for the synthesis of DNAand therefore should be used with caution in pregnant andB12-deficient patients. Nitrous oxide is also soluble in closedgas containing body spaces and can cause these spaces toenlarge when administered possibly leading to adverse occurrences(occluded middle ear, bowel distension, pneumothorax).Nitrous oxide is a popular anesthetic in dentistrywere it is commonly referred to as “laughing gas.” It is usedin combination with more potent anesthetics for surgicalanesthesia and remains a drug of recreational abuse.Nitrous oxide undergoes little or no metabolism. | | Safety Profile | Moderately toxic by
inhalation. Human systemic effects by
inhalation: general anesthetic, decreased
pulse rate without blood pressure fall, and
body temperature decrease. An experimental
teratogen. Experimental reproductive
effects. Mutation data reported. An
asphyxiant. Does not burn but is flammable
by chemical reaction and supports
combustion. Moderate explosion hazard; it
can form an explosive mixture with air.
Violent reaction with Al, B, hydrazine, LiH,
LiC6H5, PH3, Na, tungsten carbide. Also
self-explodes at high temperatures. | | Safety | Nitrous oxide is most commonly used therapeutically as an
anesthetic and analgesic. Reports of adverse reactions to nitrous
oxide therefore generally concern its therapeutic use, where
relatively large quantities of the gas may be inhaled, rather than
its use as an excipient.
The main complications associated with nitrous oxide inhalation
occur as a result of hypoxia. Prolonged administration may also be
harmful. Nitrous oxide is rapidly absorbed on inhalation. | | Potential Exposure | Used as an anesthetic in dentistry and
surgery; used as a gas in food aerosols, such as whipped
cream; used in manufacture of nitrites; used in rocket fuels;
in firefighting; diesel emissions. Large amounts of nitrous
oxide will decrease the amount of available oxygen.
Nitrous Oxide 2231
Oxygen should be routinely tested to ensure that it is at
least 19% by volume. | | Physiological effects | Nitrous oxide's primary physiological effect is
central nervous system (CNS) depression. At high concentrations, anesthetic levels can be
obtained, but the low potency of nitrous oxide
necessitates concomitant administration of other
depressant drugs. Nitrous oxide has been asso�ciated with several side effects from longterm
exposure. The most strongly substantiated effect
is neuropathy. Epidemiological studies also
suggest feto-toxic effects and higher incidents
of spontaneous abortion in exposed personnel.Although no cause-and-effect relationship has
been firmly established, exposure to the gas
should be minimized.
Inhalation of nitrous oxide without the provi�sion of a sufficient oxygen supply may be fatal
or cause brain damage. Due to the concern over
longterm exposure effects, release of the prod�uct into general work areas should be mini�mized. NIOSH has recommended a maximum exposure on an 8-hour Time-Weighted Average
(TWA) of 25 parts per million for anesthetic
and analgesic administration.
ACGIH recommends a Threshold Limit
Value-Time-Weighted Average (TLV-TWA)
of 50 ppm (90 mglm3) for nitrous oxide. The
TLV- TWA is the time-weighted average con�centration for a normal 8-hour workday and a
40-hour workweek, to which nearly all workers
may be repeatedly exposed, day after day, with�out adverse effect.
Warning: The misuse of nitrous oxide can
cause death by reducing the oxygen necessary to
support life. Nitrous oxide abuse can impair an
individual's ability to make and implement life�sustaining decisions. | | Carcinogenicity | The possible carcinogenicity of nitrous
oxide has been studied in dentists and chairside
assistants with occupational exposures. No
effect was observed in male dentists, but a 2.4-
fold increase in cancer of the cervix in heavily
exposed female assistants was reported.7 Other
epidemiological reports of workers exposed to
waste anesthetic gases have been negative.1
Carcinogenic bioassays in animals have yielded
negative results. Nitrous oxide was not genotoxic
in a variety of assays. | | storage | Nitrous oxide is essentially nonreactive and stable except at high
temperatures; at a temperature greater than 500°C nitrous oxide
decomposes to nitrogen and oxygen. Explosive mixtures may be
formed with other gases such as ammonia, hydrogen, and other
fuels. Nitrous oxide should be stored in a tightly sealed metal
cylinder in a cool, dry place. | | Shipping | UN1070 Nitrous oxide, compressed, Hazard
Class: 2.2; Labels: 2.2-Nonflammable compressed gas;
5.1-Oxidizer; UN2201 Nitrous oxide, refrigerated liquid,
Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed
gas; 5.1-Oxidizer. Cylinders must be transported in a secure
upright position, in a well-ventilated truck. Protect cylinder
and labels from physical damage. The owner of the compressed
gas cylinder is the only entity allowed by federal
law (49CFR) to transport and refill them. It is a violation
of transportation regulations to refill compressed gas cylinders
without the express written permission of the owner. | | Purification Methods | Wash the gas with concentrated alkaline pyrogallol solution, to remove O2, CO2, and NO2, then dry it by passing it through columns of P2O5 or Drierite, and collecting in a dry trap cooled in liquid N2. It is further purified by freeze-pump-thaw and distillation cycles under vacuum [Ryan & Freeman J Phys Chem 81 1455 1977, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 484-485 1963]. | | Toxicity evaluation | Large amounts of released nitrous oxide can decrease the
amount of available oxygen. Medical complications of nitrous
oxide inhalation are due to varying degrees of hypoxia affecting
primarily the heart and brain. By inactivating vitamin B12,
a critical cofactor in hematopoiesis and lipid membrane
formation, nitrous oxide can cause anemia and neuropathy via
selective inhibition of methionine synthase, a key enzyme in
methionine and folate metabolism. | | Incompatibilities | Nitrous oxide is generally compatible with most materials
encountered in pharmaceutical formulations, although it may react
as a mild oxidizing agent. | | Incompatibilities | Nitrous oxide is a weak oxidizer.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoacids,
epoxides. Violent reactions with organic peroxides,
hydrazine, hydrogen, hydrogen sulfide; lithium, boron, lithium
hydride, sodium, aluminum, phosphine. This chemical
is a strong oxidizer @ .300�C and self-explodes at high
temperature. May form explosive mixtures with ammonia,
carbon monoxide; hydrogen sulfide; oil, grease and fuels. | | Waste Disposal | Disperse in atmosphere or
spray on dry soda ash/lime with great care; then flush to
sewer. | | Regulatory Status | GRAS listed. Accepted for use as a food additive in Europe.
Included in nonparenteral medicines licensed in the UK and USA.
Included in the Canadian List of Acceptable Non-medicinal
Ingredients. | | GRADES AVAILABLE | Nitrous oxide is available in medical, commer�cial, and high-purity grades. The medical (USP)
grade is the most widely used. Manufacturers
typically produce nitrous oxide for this use to
the specification published in the United States
Pharmacopeia/National Formulary. CGA
G-8.2, Commodity Specification for Nitrous
Oxide, describes the requirements for particular
grades of nitrous oxide. Other specifications
to meet particular requirements are available
from suppliers. The absence of a
value in a listed quality verification level does
not mean to imply that the limiting characteristic is or is not present, but merely indicates that
the test is not required for compliance with the
specification. |
| | NITROUS OXIDE Preparation Products And Raw materials |
|