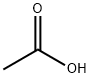
Acetic acid polarity and related applied research
Dec 19,2023
Acetic acid polarity
Acetic acid is a hydrophilic (polar) solvent commonly used in laboratories, similar to ethanol and water. It has a relative static dielectric constant (dielectric constant) and a relative polarity value of 6.2 and 6.4, respectively, and can dissolve not only polar compounds such as inorganic salts and sugars, but also non-polar compounds such as oils and polar solutes. It is miscible with polar and non-polar solvents such as water, chloroform and hexane.

For the higher alkanes (from octane), acetic acid is immiscible in all combinations, and the solubility of acetic acid in alkanes decreases with prolongation of the n-alkanes. The solvent and miscibility of acetic acid makes it a useful industrial chemical, for example, as an extractant or as a solvent in the production of dimethyl terephthalate.
Related Applied Research
Pervaporation of water–alcohol mixtures and acetic acid–water mixtures
In this study, pervaporation experiments were performed with methanol–water mixtures, ethanol–water mixtures, IPA (isopropyl alcohol)–water mixtures, and acetic acid–water mixtures, over the complete concentration range allowed by the membrane. The results of the three water–alcohol mixtures are compared to investigate the influence of molecular weight and polarity on the permeation behavior.
The results show that ethanol and IPA have a similar permeation behavior, whereas methanol shows a different behavior. This can be explained by the relatively high polarity of methanol, which makes methanol sorption competitive with water sorption. The IPA–water mixtures are compared with the acetic acid–water mixtures. IPA and acetic acid have approximately the same molecular weight, but contain a different functional group. The acetic acid–water mixtures have a higher total flux than the IPA–water mixtures.
Moreover, the partial acetic acid flux is higher than the partial IPA flux. Acetic acid contains a carbonyl group, which has a high capacity of forming hydrogen bonds with the alcohol groups of the PVA (polyvinyl alcohol) top layer. Moreover, it is a large molecule, causing high swelling resulting in a high permeation flux and a low separation factor. The behavior of the acetic acid–water mixtures shows more resemblance to the methanol–water mixtures than to the IPA–water mixtures. This suggests that the polarity and functional group of the different feed components is more important than the molecular size.
Influence of the polarity of the organic phase on the reactive extraction of alkaloids
The reactive extractions of acetic acid, HAc, with tri-n-octylamine (TOA), Q, dissolved in three solvents with different dielectric constants (dichloromethane, butyl acetate, and n-heptane) without and with 1-octanol as phase modifier have been comparatively analyzed. The results indicated that the mechanism of the interfacial reaction between acid and extractant is controlled by the organic phase polarity. In absence of 1-octanol, the structures of the extracted complexes are HAc.Q for dichloromethane, HAc.Q2 for butyl acetate, and (HAc)2Q4 for n-heptane. These structures are modified by adding 1-octanol and become HAc.Q for extraction in dichloromethane or butyl acetate and (HAc)2Q2 for extraction in n-heptane, respectively. Although the presence of 1-octanol improves the extraction efficiency, it leads to the reduction of extraction constants for lower-polar solvents, influence that is more significant for n-heptane.
References:
[1] D. VAN BAELEN. Pervaporation of water–alcohol mixtures and acetic acid–water mixtures[J]. Chemical Engineering Science, 2005, 60 6: Pages 1583-1590. DOI:10.1016/j.ces.2004.10.030.
[2] DAN CAŞCAVAL*; Anca I G; Lenuţa Kloetzer. Influence of Organic Phase Polarity on Interfacial Mechanism and Efficiency of Reactive Extraction of Acetic Acid with Tri-n-octylamine[J]. Journal of Chemical & Engineering Data, 2011, 56 5: 2521-2526. DOI:10.1021/je200044y.
- Related articles
- Related Qustion
- What is the Composition of Vinegar and is it Acidic or Alkaline? Feb 28, 2024
Vinegar offers numerous health benefits and enhances the flavor of dishes, making it a kitchen essential. This article will introduce the composition and application of it.
- Acetic Acid - Application in Meat Processing & Food Industry Mar 10, 2022
Acetic or ethanoic acid is a monocarboxylic acid which occurs naturally in plant and animal tissues and is also a byproduct of ethanol oxidation by Acetobacter , Gluconobacter and other heterofermentative strains of lactic acid bacteria.
- What is Acetic acid? Oct 13, 2021
Acetic acid is present throughout nature as a normal metabolite of both plants and animals. Acetic acid may also be released to the environment in a variety of waste effuents, in emissions from combustion processes, and in exhaust from gaso
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringEthyl acetate is a moderately polar compound containing a non-polar ethyl group, a polar carbonyl (C=O) group and a polar oxygen atom.....
Dec 19,2023APIAcetic acid
64-19-7You may like
- Glacial acetic acid
-

- $1.00 / 1KG
- 2025-12-11
- CAS:64-19-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10mt
- Acetic Acid
-
- $0.00 / 200kg
- 2025-11-19
- CAS:64-19-7
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Acetic acid
-

- $1117.00 / 200kg
- 2025-09-26
- CAS:64-19-7
- Min. Order: 200kg
- Purity: 99.99%
- Supply Ability: 100Tons