Allopurinol: the treatment of elevated uric acid levels
Jan 2,2024
Introduction
Allopurinol is a medication widely employed in the treatment of elevated uric acid levels, notably in the management of gout, a form of arthritis marked by painful joint inflammation due to uric acid crystal deposition. Operating by inhibiting the enzyme xanthine oxidase, allopurinol hinders the conversion of hypoxanthine to xanthine and subsequently to uric acid, thus reducing uric acid production and preventing crystal formation. Administered orally, dosage is individualized based on the severity of the condition, with regular monitoring of uric acid levels to gauge treatment effectiveness. While generally well-tolerated, common side effects include rash and gastrointestinal disturbances, and precautions are necessary in individuals with kidney or liver impairments. The rare but serious hypersensitivity syndrome underscores the importance of careful monitoring and prompt medical attention. In essence, allopurinol proves to be a pivotal medication in mitigating the symptoms and preventing the recurrence of conditions associated with elevated uric acid levels.
Application
1. Gout is a kind of inflammatory arthritis with a high incidence rate in clinic, which is caused by intra-articular urate crystals. It has a certain correlation with cardiovascular disease, chronic kidney disease, etc. Men and postmenopausal women are the high incidence groups of this disease. Based on the later symptoms of gout patients, many scholars regard it as a disease caused by excessive uric acid, often accompanied by widespread complications and arthritis. Hyperuricemia, gout stones accumulation, and arthritis deformities are the main characteristic manifestations of this disease. When people of working age suffer from gout disease, their work attendance rate will correspondingly decrease, and their productivity and physical function will be reduced to varying degrees. Based on the latest pathological and physiological explanations, gout is two interrelated diseases with certain differences in process: one is the formation of uric acid in the body, but when its level rises to a certain level, it will promote the aggregation and precipitation of monosodium urate into a crystalline state; The second is the inflammatory response caused by monosodium urate crystals, which is mostly in patients with acute gout. In the past, clinical practice has achieved a reduction in serum urate concentration by inhibiting the body's uric acid synthesis process, and it is often recommended to use allopurinol and febuxostat. In the past decade, many scholars have provided dynamic support for new and specific therapeutic targets by studying the pathogenesis of gout, and finally developed the first uric acid lowering therapy applied in more than 40 years, with febuxostat as the leading drug. The molecular structure of febuxostat contains hydrogen bonds, salt bridges, and hydrophobic amino acid active ends, which basically completely fill the channel leading to the XO center, hindering the XO binding to its receptor process, and selectively inhibiting the biological activity of XO, ultimately reducing the production of uric acid in the body5.
2. Cardiovascular disease (CVD) remains the leading cause of death in the United States. There is evidence that shows a direct relationship between an elevated uric acid level and an increased risk of cardiovascular (CV) events, which has set the foundation for the investigation of uric acid-lowering drugs for the treatment of CVD. Although traditionally the cornerstone therapy for gout, allopurinol's ability to be a competitive inhibitor of the key enzyme, xanthine oxidase, needed for uric acid formation, has prompted recent clinical research evaluating allopurinol as a CV drug. Epidemiologic and biochemical studies on uric acid formation have shown that it is not only uric acid itself that leads to worsening prognosis and increased CV events, but also the free radicals and superoxides formed during xanthine oxidase activity. The combination of uric acid formation and formed free radicals could ultimately lead to coronary endothelial dysfunction and worsening of myocardial oxidative stress. Along with preventing uric acid formation, allopurinol also has the ability to behave as a free radical scavenger of the superoxide anions and free radicals released during uric acid formation. Clinical studies have shown that allopurinol improves endothelial dysfunction and subsequently improves the exercise capacity in patients diagnosed with angina pectoris. Allopurinol has also been shown to decrease oxidative stress and ameliorate the morbidity and mortality of congestive heart failure patients by possibly improving mechanoenergetic uncoupling, with the enhancement of myocardial contractility and the left ventricular ejection fraction3.
Synthesis
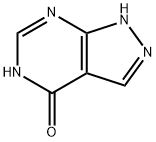
Allopurinol is a marketed drug for treating gout, and the optimization of allopurinol production processes has been a focus of industrial research. In the original process, both formamide and formic acid were required, and allopurinol was generated at a reaction temperature of 180°C. The optimized process, however, only requires formamide (or formic acid) to produce allopurinol, with the reaction temperature reduced to around 100°C, resulting in a maximum yield of 89%. This optimization significantly reduces the raw material cost of the reaction, saves energy consumption, and further ensures the safety of industrial production. Glycosylation reactions typically require anhydrous and anaerobic conditions, imposing limitations on their development. This study aims to develop a novel and operationally simple glycosylation reaction route, specifically generating arabino-carbohydrate glycosides/arabino-oxygen glycosides in an open system using cesium carbonate and 1,4-bis(diphenylphosphino)butane (DPPB). Regarding oxygen glycosylation synthesis strategies, in-depth research was conducted. Mechanistic studies indicate that chemical/stereo selectivity arises from the coordination of phenol, generated in situ from arylboronic acid oxidation, with a palladium catalyst, followed by intramolecular nucleophilic attack. Isotope labeling experiments suggest that the oxygen element in the oxygen glycosidic bond comes from O2. The practicality of this glycosylation strategy was further demonstrated through the synthesis and modification of natural products and the functionalization of unsaturated oxygen glycoside products. In 1976, Shanghai Ninth Pharmaceutical Factory in China first used ethyl cyanoacetate as a raw material to organize the production of allopurinol products. This process route includes the condensation process of ethyl cyanoacetate and triethyl orthoformate to obtain ethoxymethylene cyanoacetate ethyl ester, the ammonia water cyclization pyrazole ring process, and the formamide cyclization allopurinol process. This synthesis route requires two cyclization cycles, with a total yield of 46%. In 1978, Wang Yongxiao and others proposed an improvement plan based on this synthesis, as shown in Figure 14.
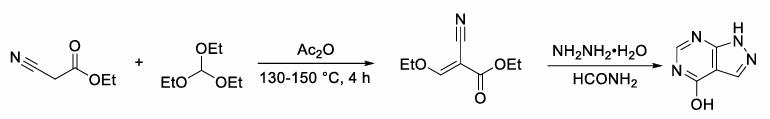
Figure 1 Synthesis of allopurinol using ethyl cyanoacetate method
Safety
Allopurinol is the most commonly used urate lowering therapy in the management of gout. Despite the fact that it has been available for over 40 years there is ongoing debate about optimal allopurinol dosing in gout patients with chronic kidney disease. Given that gout is common in patients with renal impairment, clinicians need to be aware of the relationships between serum urate and kidney function as well as the effects of allopurinol on kidney function and vice versa. The use of allopurinol in patients on dialysis is an understudied area. Dialysis reduces plasma oxypurinol concentrations, therefore the dose and time of administration in relationship to dialysis need to be carefully considered. Recently, it has been suggested that there may be a role for allopurinol in patients with chronic kidney disease without gout. Observational studies have reported an association between serum urate and chronic kidney disease and end stage renal failure. The effect of urate lowering therapy with allopurinol on progression of kidney disease has been examined in small studies with varying results. Larger clinical trials are currently underway2. Hyperuricemia is present in approximately 5% of the population, the vast majority of whom are asymptomatic and at no clinical risk. Complications, including renal calculi, uric acid nephropathy and gout, occur in a small proportion of patients. Allopurinol, an analog of hypoxanthine, has been widely used in clinical practice for over 30 years for the treatment of hyperuricemia and gout. Two percent of patients taking this medication develop a mild exanthema. A syndrome characterized by exfoliative dermatitis, hepatitis, interstitial nephritis and eosinophilia has been described previously. Termed allopurinol hypersensitivity syndrome, its etiology is related to the accumulation of one of the allopurinol metabolites, oxypurinol; clearance of oxypurinol is decreased in the setting of renal insufficiency and the use of thiazide diuretics. The term DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms) was recently introduced to describe a disorder associated with various drugs or viral infections and characterized by similar features. The pathophysiology of allopurinol-induced hypersensitivity, clinical presentation and treatment are reviewed1.
Reference
1. Markel A. Allopurinol-induced DRESS syndrome. Isr Med Assoc J. 2005 Oct;7(10):656-60.
2. Stamp LK, Chapman PT, Palmer SC. Allopurinol and kidney function: An update. Joint Bone Spine. 2016 Jan;83(1):19-24.
3. Kelkar A, Kuo A, Frishman WH. Allopurinol as a cardiovascular drug. Cardiol Rev. 2011 Nov-Dec;19(6):265-71.
4. Wang Qiuyuan Optimization of the synthesis process of allopurinol and stereoselective synthesis of arabinose [D]. Three Gorges University, 2023.
5. Zhang Lin, Liu Linna, Shi Jieli, et al. Progress in the synthesis of anti gout drug febuxostat [J]. Chinese Prescription Medicine, 2021,19 (05): 23-25.
- Related articles
- Related Qustion
- Allopurinol: A Comprehensive Overview for Chemistry Professionals May 14, 2024
Allopurinol is a medication commonly used in the treatment of gout and certain types of kidney stones.
- Allopurinol: mechanism of action and clinical efficacy Jun 20, 2023
Allopurinol is a medication commonly used to treat gout. However, recent studies have shown that Allopurinol may be beneficial for other health conditions as well.
- Allopurinol - Reaction / Application on synthetic works Nov 19, 2019
Allopurinol is xanthine oxidase-activated prodrug of thymidine phosphorylase inhibitor. It is also an important organic intermediate to synthetize substituted pyrimidine products.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringUrolithin A, derived from foods like pomegranates, shows potential in promoting health, mitigating diseases, and enhancing longevity.....
Jan 2,2024Natural ProductsAllopurinol
315-30-0You may like
- Allopurinol
-

- $0.00 / 25Kg/Drum
- 2025-12-05
- CAS:315-30-0
- Min. Order: 1KG
- Purity: 98%-102%;USP
- Supply Ability: 10 TONS
- Allopurinol
-
- $65.00 / 10g
- 2025-12-04
- CAS:315-30-0
- Min. Order:
- Purity: 99.36%
- Supply Ability: 10g
- Allopurinol
-
- $65.00 / 10g
- 2025-12-04
- CAS:315-30-0
- Min. Order:
- Purity: 99.36%
- Supply Ability: 10g