Divinyltetramethyldisiloxane: Roles in Metal Complex Synthesis and Platinum Recovery
Sep 25,2025
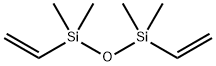
Divinyltetramethyldisiloxane is a versatile reagent with applications in organometallic synthesis, particularly in forming metal complexes and as a precursor in silylative couplings1. It is widely used as a ligand to form metal precatalysts with Pt, Pd, Ni, Rh, etc. DVDS is an inexpensive, less toxic, and more accessible vinyl cross-coupling reagent. The Hiyama cross-coupling of divinyltetramethyldisiloxane with various iodo- and bromoarenes in the presence of catalyst affords functionalized styrenes. The reagent is a moisture-sensitive, colorless oil that hydrolyzes in water slowly. It should be stored under anhydrous conditions and is incompatible with strong oxidizers and halogens. It is suitable for use as an additive (intermediate) in the production processes of addition-type silicone rubber, silica gel, liquid silicone rubber, vinyl-based silicone resin, vinyl-based silicone oil, platinum-chromium compounds, etc. It is a type of high-performance functional organosilicon material that can be used as an important auxiliary agent in olefin polymerization catalysts to improve polymerization yield, reaction selectivity, and product performance.

(Aminocarbene)(Divinyltetramethyldisiloxane)Iron(0) Compounds
Low-valent transition-metal alkene complexes are valuable organometallic species owing to their wide applications as catalysts in organic synthesis and as precursors for preparing new complexes. Along with growing interests in base metal chemistry in the recent years, low-valent iron alkene compounds have been subjected to extensive investigation. While these studies focused on low-spin 17- and 18-electron poly(alkene) species, we report herein a class of unprecedented high-spin 16- and 14-electron iron(0) alkene complexes supported by aminocarbene ligands, [LnFe(dvtms)] (L=N-heterocyclic carbene (NHC) or cyclic (alkyl)(amino)carbene (CAAC), n=1 or 2, dvtms=divinyltetramethyldisiloxane). These iron(0) alkene complexes are readily accessible. Their propensity to undergo oxidation, as demonstrated by the reactions with Ph2SiH2, S8, Se, and DippN3 to form novel aminocarbene-supported (silylene)iron(IV), iron(II)–sulfur/selenium cubanes, as well as bis(imido)iron(IV) compounds, shows their potential synthetic application. The addition of KC8 or Na/Hg (two equiv) to the mixtures of FeCl2, Divinyltetramethyldisiloxane, and the corresponding free aminocarbene ligands (one or two equiv) produced dark blue suspensions, from which air- and moisture-sensitive green or greenish blue crystals of [(IEt2Me2)2Fe(dvtms)], [(IMes)Fe(dvtms)], and [(Me2-CAAC)Fe(dvtms)] (IEt2Me2=1,3-diethyl-4,5-dimethylimidazol-2-ylidene, IMes=1,3-di(2′,4′,6′-trimethylphenyl)imidazol-2-ylidene, Me2-cAAC=1-(2′,6′-diisopropylphenyl)-3,3,5,5-tetramethyl- pyrrolidine-2-ylidene) were isolated in 44–87 % yields.[1]
Complexes have been characterized by 1H NMR spectroscopy, solution magnetic susceptibility measurement, UV/Vis-NIR spectroscopy, infrared resonance, single-crystal X-ray diffraction, 57Fe Mössbauer spectroscopy, and elemental analysis. In conclusion, we have prepared and characterized novel 16- and 14-electron high-spin iron(0) alkene compounds by using aminocarbene and divinyltetramethyldisiloxane as ancillary ligands. These coordinatively unsaturated iron(0) compounds can serve as useful synthons for [L2Fe0] and [LFe0] (L=aminocarbene) complexes, which has been demonstrated by their reactions with hydrosilanes, elemental sulfur/selenium, and organic azides to produce aminocarbene-supported (silylene)iron(IV), all-ferrous iron–sulfur/selenium cubanes, as well as mononuclear bis(imido)iron(IV) compounds. Further exploration of the reactivity and catalytic application of these aminocarbene-supported iron(0) alkene complexes are underway.
Rapid and Efficient Collection of Platinum from Karstedt’s Catalyst Solution
Hydrosilylation reactions in which carbon–silicon bonds are created are very important and extensively used in academic research and in the silicone industry. Some examples of the chemistry and applications of these kinds of reactions include (1) hydrosilylation of carbon–carbon multiple bonds in the synthesis of molecular organosilicon compounds, such as silane coupling agents and UV screens; (2) chemo- and enantioselective hydrosilylation of unsaturated carbon–heteroatom bond. Normally, Karstedt’s catalyst is used for the hydrosilylation reactions after dilution to a certain concentration in the range of 10–100 ppm using a divinyltetramethyldisiloxane ligand-compatible solvent, such as tetrahydrofuran, ethyl acetate, xylene, or vinyl-terminated poly(dimethyl silicone) (Vi-PDMS), or others. The major role of Karstedt’s catalyst is attributed to platinum, which is one of the rarer elements and both highly valuable and precious. Herein, as a first attempted example so far, we report a facile and efficient collection and precise evaluation of Pt from samples of the Karstedt’s catalysts taking advantage of a ligand-exchange reaction between divinyltetramethyldisiloxane ligand and propynol.[2]
Our strategy is developed from our occasional findings. In particular, during attempts to prepare silicone release coatings from Vi-PDMS and poly(methyl hydrosiloxane) in the presence of the Karstedt’s catalyst, we have sometimes mistakenly added excessive propynol to the catalyst. Black precipitates are observed surrounding the propynol droplets 1–2 min after the addition of propynol. These precipitates are believed to contain Pt, and they are apparently formed by a ligand-exchange reaction between propynol and divinyltetramethyldisiloxane ligand. This phenomenon inspired us to collect Pt belonging to the Karstedt’s catalyst from these precipitates. In summary, we have developed a facile and efficient strategy for the collection and precise evaluation of Pt from the samples of the Karstedt’s catalysts. The propynol reacted with platinum via a ligand-exchange reaction between divinyltetramethyldisiloxane ligand and propynol. The Karstedt’s catalyst underwent reconstruction and assembled into larger particles due to hydrogen bonding among the hydroxyl groups of the propynol ligands. The resultant large particles were readily soluble in water and thus facilitated the collection of Pt from the Karstedt’s catalyst. The efficient collection of Pt from the Karstedt’s catalyst was confirmed by performing the reaction between propynol and Karstedt’s catalyst at various concentrations.
Synthesis and behavior of N-heterocyclic carbene (η4-diene) platinum(0) complexes
The chemistry of N-heterocyclic carbenes (NHCs) has flourished over the past few decades, mainly due to their outstanding properties as ancillary ligands. As NHCs offer a unique combination of properties, such as thermal and oxidative stability, strong σ-donating character, and electronic and steric tunability due to their versatile substitution, they usually provide steric protection and bind strongly to metal centers, thus resulting in complexes that are very often stable to air and moisture. A series of water-soluble (NHC)Pt(0)(dvtms) and (NHC)Pt(0)(AE) complexes containing different sulfonated NHC ligands (dvtms = divinyltetramethyldisiloxane and AE = diallyl ether) are reported. The divinyltetramethyldisiloxane compounds have been found to be quite robust and to display some conformational rigidity, whereas their AE counterparts are less stable and more flexible. The catalytic evaluation of these complexes in the hydrosilylation of alkynes in water revealed no benefits in favor of the complexes containing the more labile spectator diene (AE), and a fairly regular catalytic behavior for all complexes that restricts the location of the sulfonate group to the proximity of the metal site.
References
[1]Zhang H, Ouyang Z, Liu Y, Zhang Q, Wang L, Deng L. (Aminocarbene)(divinyltetramethyldisiloxane)iron(0) compounds: a class of low-coordinate iron(0) reagents. Angew Chem Int Ed Engl. 2014 Aug 4;53(32):8432-6. doi: 10.1002/anie.201404677. Epub 2014 Jun 24. PMID: 24961207.
[2]Yang G, Wei Y, Huang Z, Hu J, Liu G, Ou M, Lin S, Tu Y. Rapid and Efficient Collection of Platinum from Karstedt's Catalyst Solution via Ligands-Exchange-Induced Assembly. ACS Appl Mater Interfaces. 2018 Feb 21;10(7):6778-6784. doi: 10.1021/acsami.7b19644. Epub 2018 Feb 8. PMID: 29381049.
[3]Ruiz-Varilla AM, Baquero EA, Silbestri GF, Gonzalez-Arellano C, de Jesús E, Flores JC. Synthesis and behavior of novel sulfonated water-soluble N-heterocyclic carbene (η(4)-diene) platinum(0) complexes. Dalton Trans. 2015 Nov 14;44(42):18360-9. doi: 10.1039/c5dt02622a. Epub 2015 Sep 8. PMID: 26346995.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringFluoxymesterone treats low testosterone/delayed puberty/breast cancer; it aids Tam in AR-enriched breast cancer and inhibits human 11β-HSD2.....
Sep 26,2025APIDivinyltetramethyldisiloxane
2627-95-4You may like
Divinyltetramethyldisiloxane manufacturers
- Divinyltetramethyldisiloxane
-

- $0.00 / 5kg
- 2025-12-12
- CAS:2627-95-4
- Min. Order: 0.001kg
- Purity: 99%
- Supply Ability: 200000kg
- Divinyltetramethyldisiloxane
-

- $1.00 / 200KG
- 2025-12-12
- CAS:2627-95-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1000mt/year
- Divinyltetramethyldisiloxane
-
- $100.00 / 25KG
- 2025-12-11
- CAS:2627-95-4
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 200TON