How does NBS work in organic chemistry?
Apr 17,2024
Description
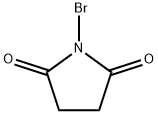
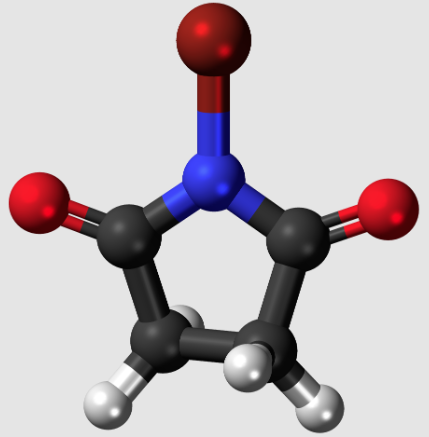
N-bromosuccinimide is a five-membered cyclic dicarboximide compound having a bromo substituent on the nitrogen atom. It is a dicarboximide, a pyrrolidinone, and an organobromine compound. It is functionally related to a succinimide.
Uses
It is an organobromide compound used in various bromination reactions for chemical synthesis. It can donate bromine to alkenes, allylic or benzyl compounds, carbonyl-containing compounds, and aromatic compounds such as phenols, anilines, and various aromatic heterocycles. N-Bromosuccinimide will decompose over time, giving off bromine. Pure NBS is white, but it is often found to be off-white or brown-colored by bromine. NBS can be used as a protein modification agent that targets tryptophan and histidine residues.
Reaction
N-Bromosuccinimide is a brominating and oxidizing agent that is used as a source of bromine in radical reactions (for example, allylic brominations) and various electrophilic additions. It will do many of the same reactions as bromine – attached to the electron-withdrawing nitrogen of succinimide, the bromine has a partial positive charge and is, therefore, electrophilic. The NBS bromination of substrates such as alcohols and amines, followed by the elimination of HBr in the presence of a base, leads to the products of net oxidation in which no bromine has been incorporated.
NBS is an inexpensive, commercially available, and versatile reagent. This reagent has recently been used as an effective catalyst for the acetalization of carbonyl compounds, the conversion of aldehydes to 1,1-diacetates, and the acylation of alcohols under mild and nearly neutral reaction conditions. N-Bromosuccinimide catalyzes the ring opening of various epoxides with different thiols in CH3CN at room temperature under mild reaction conditions. The corresponding β-hydroxysulphides are obtained in short reaction times and good to high yields with nearly complete regioselectivity[1].
Reference
[1] Rostami, Amin , and H. Jafari . "NBS as a powerful catalyst for the synthesis of β-hydroxysulphides with thiolysis of epoxides under mild reaction conditions : research article." South African journal of chemistry. Suid-Afrikaanse tydskrif vir chemie 61(2008):115-118.
- Related articles
- Related Qustion
- N-Bromosuccinimide:Chemical synthesis,Reactions Mar 20, 2023
N-Bromosuccinimide (NBS) is a convenient source of bromine for both radical substitution and electrophilic addition reactions.
- What is N-Bromosuccinimide? Jul 2, 2020
N-Bromosuccinimide is five-membered cyclic dicarboximide compound having a bromo substituent on the nitrogen atom. N-Bromosuccinimide (NBS) has been extensively used both in the bromination and oxidation of many classes of organic compounds
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringIodomethane is used as an intermediate in manufacturing some pharmaceuticals and pesticides.....
Apr 17,2024Organic reagentsN-Bromosuccinimide
128-08-5You may like
N-Bromosuccinimide manufacturers
- N-Bromosuccinimide
-

- 2025-12-11
- CAS:128-08-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- N-Bromosuccinimide
-
- $1.00 / 1PCS
- 2025-12-11
- CAS:128-08-5
- Min. Order: 1PCS
- Purity: 99%
- Supply Ability: 10 mt
- N-Bromosuccinimide
-

- $0.00 / 25KG
- 2025-12-11
- CAS:128-08-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000mt