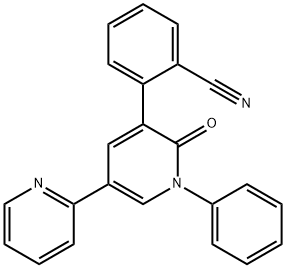
Mechanism of action and pharmacokinetics of pyrenoprene
Aug 15,2019
INTRODUCTION
Epilepsy is a serious neurological condition that affects more than 50 million individuals globall.The Food and Drug Administration approved perampanel (Fycompa, Eisai, Inc.) in October 2012 as an adjunctive agent for the treatment of POS with or without secondary generalization in patients with epilepsy at least 12 years of age. In June 2015, the agency approved a second indication for primary generalized tonic-clonic (PGTC) seizures in patients with epilepsy who are at least 12 years of age.
MECHANISM OF ACTION
Perampanel (2-[2-oxo-1-phenyl-5-pyridin-2-yl-1,2 dihydropyridin-3-yl] benzonitrile hydrate) is a novel non-competitive selective antagonist at the postsynaptic ionotropic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor.1,8,9 In the nervous system, glutamate is known to be a major excitatory neurotransmitter, but the exact antiepileptic mechanism of perampanel in humans is unknown.8 Studies suggest that AMPA receptor antagonism can lead to reduced overstimulation and anticonvulsant effects, as well as inhibiting seizure generation and spread. In addition, AMPA receptor antagonists may prevent neuronal death.
PHARMACOKINETICS
Absorption
Administration of perampanel results in rapid and complete absorption with negligible first-pass metabolism. The median time to reach peak concentration varies between 0.5 to 2.5 hours fasting (delayed by two to three hours when taken with food). Peak plasma concentration is reached in approximately one hour (decreased by 40% when taken with food). It is worth noting that the extent of absorption is not affected by food.
Distribution
Fycompa is approximately 95% to 96% protein-bound in the concentration range of 20 ng/mL to 2,000 ng/mL.
CLINICAL TRIALS
Clinical trials of perampanel have been conducted with patients diagnosed with PGTC seizures, and with those undergoing uncontrolled, drug-resistant, or refractory POS. In all studies, the primary efficacy endpoint was the percent change in seizure frequency per 28 days, and a common secondary efficacy endpoint was the 50% responder rate. In the 2015 clinical trial conducted by French et al., patients with PGTC seizures who were taking 8 mg or the highest tolerated dose of perampanel showed a statistically significant reduction in the frequency of seizures compared with placebo.
- Related articles
- Related Qustion
- Perampanel: A Promising AMPA Receptor Antagonist for Epilepsy Treatment Nov 12, 2024
Perampanel is used alone or together with other medicines to treat certain types of epilepsy, such as partial onset seizures and generalized tonic-clonic seizures.
- What are the side effects of PeraMpanel? Dec 21, 2022
Perampanel is used to treat certain types of seizures (including focal and generalized tonic-clonic seizures). Perampanel belongs to a class of drugs known as anticonvulsants.
Eplerenone is the second oral aldosterone antagonist available for the treatment of essential hypertension and heart failure. Treatment has been associated with reductions in blood pressure and improved survival (15% reduction in total mortality) for patients with heart failure who are in stable condition after a myocardial infarction.....
Aug 15,2019APICannabidiol is a chemical in the Cannabis sativa plant, also known as marijuana or hemp. Over 80 chemicals, known as cannabinoids, have been identified in the Cannabis sativa plant. While delta-9-tetrahydrocannabinol (THC) is the major active ingredient in marijuana, cannabidiol is also obtained from hemp, which contains only very small amounts of THC.....
Aug 15,2019Natural ProductsPeraMpanel
380917-97-5You may like
- PeraMpanel
-
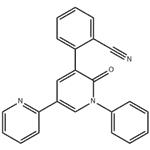
- $0.00 / 1kg
- 2025-09-16
- CAS:380917-97-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100kg
- PeraMpanel
-
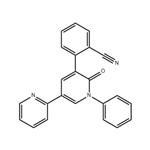
- $0.00 / 25kg
- 2024-03-28
- CAS:380917-97-5
- Min. Order: 25kg
- Purity: 98%-99%
- Supply Ability: Inquiry
- Perampanel-D5
-
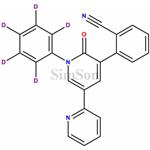
- $0.00 / 5mg
- 2023-05-27
- CAS:
- Min. Order: 1mg
- Purity: 96%
- Supply Ability: 50 mg