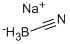Sodium Cyanoborohydride: A Versatile Agent in Chemical Synthesis
May 23,2024
Introduction
Sodium cyanoborohydride (NaCNBH₃) is a chemical compound extensively utilized in the realm of organic synthesis. Known for its selectivity and stability, this agent offers a plethora of applications, particularly in the reduction of imines to amines, a crucial step in the synthesis of numerous pharmaceuticals and fine chemicals. Its ability to facilitate reactions under mild conditions while maintaining excellent yields makes it a preferred choice among chemists. Additionally, NaCNBH₃ is celebrated for its role in reducing unwanted side reactions, thereby enhancing the overall efficiency and purity of the chemical processes. This characteristic is especially valuable in the synthesis of sensitive and complex molecules, where precision and control are paramount. Thus, sodium cyanoborohydride stands as a vital tool in the arsenal of modern synthetic chemistry, enabling innovations and advancements in drug development and materials science[1].

Figure 1 Characteristics of SODIUM CYANOBOROHYDRIDE
Synthesis
The synthesis of sodium cyanoborohydride is typically carried out through the reaction of sodium borohydride (NaBH₄) with hydrogen cyanide (HCN), under controlled conditions. This process involves careful manipulation of reactant ratios and temperatures to ensure the formation of NaCNBH₃ while minimizing side reactions. The purity of the product is crucial, as trace impurities can significantly affect its reactivity and safety profile.
Main Components
Sodium cyanoborohydride consists primarily of sodium (Na), boron (B), hydrogen (H), and cyanide (CN). Each of these elements plays a vital role in the chemical behavior of the compound. The cyanide group, in particular, imparts unique characteristics that differentiate NaCNBH₃ from other borohydrides, such as increased stability towards protic solvents. This stability is pivotal in applications that require selective reduction in the presence of sensitive functional groups. The boron atom in the molecule provides the basis for its reductive capabilities, making it an excellent source of hydride ions that are essential for the reduction reactions. Additionally, the sodium ion helps in stabilizing the molecular structure, facilitating its handling and use in various organic solvents. This combination of elements not only enhances the reactivity of NaCNBH₃ but also contributes to its safe and effective application in diverse chemical synthesis processes.
Applications
The applications of sodium cyanoborohydride are vast and varied. One of its most prominent uses is in the reductive amination of aldehydes and ketones, a key method for the synthesis of amines from carbonyl compounds. This reaction is fundamental in the production of pharmaceuticals, where the introduction of amine groups is often necessary for biological activity.
Furthermore, NaCNBH₃ is employed in the reduction of acyl derivatives and the selective reduction of carbon-carbon double or triple bonds adjacent to a cyano group. This selectivity is particularly useful in complex multi-step syntheses where the preservation of other functional groups is critical. In addition to its synthetic applications, sodium cyanoborohydride is also used in DNA and RNA research, serving as a reducing agent in the modification and labeling of nucleic acids.
Storage
The storage of sodium cyanoborohydride requires specific conditions to maintain its stability and reactivity. It should be kept in a cool, dry place, away from moisture and direct sunlight. NaCNBH₃ is also sensitive to air, and prolonged exposure can lead to degradation and a reduction in efficacy. Therefore, it is typically stored under an inert atmosphere in tightly sealed containers. Proper handling and storage procedures are essential to ensure the safety and integrity of the compound.
Conclusion
Sodium cyanoborohydride is a cornerstone chemical in the field of organic synthesis, revered for its versatility and efficiency. From pharmaceuticals to research applications, its role in facilitating complex chemical transformations is undeniable. Understanding the synthesis, properties, and safe handling of NaCNBH₃ is crucial for chemists and professionals working with this valuable reagent. With ongoing research and development, the potential applications and methodologies utilizing sodium cyanoborohydride continue to expand, further solidifying its importance in the scientific community[2].
![]() References
References
[1]Clinton F. Sodium cyanoborohydride—a highly selective reducing agent for organic functional groups[J]. Synthesis, 1975, 3: 135-146.
[2]Han O, Shih Y, Liu L, et al. On the mechanism of sodium cyanoborohydride reduction of tosylhydrazones[J]. The Journal of Organic Chemistry, 1988, 53(9): 2105-2108.
- Related articles
- Related Qustion
- One of the reductants for reductive amination: sodium cyanoborohydride May 8, 2024
Sodium cyanoborohydride is a selective reducing agent used for various chemical reductions, including aldehydes, ketones, oximes, enamines, reductive aminations of aldehydes and ketones, and reductive alkylations of amines and hydrazines.
- What is Sodium cyanoborohydride? Sep 19, 2023
Sodium cyanoborohydride is a mild reducing agent that is commonly used for a variety of chemical reductions
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringIn the realm of organic chemistry, esters have always played a pivotal role due to their versatile applications across various industries.....
May 23,2024APISodium cyanoborohydride
25895-60-7You may like
Sodium cyanoborohydride manufacturers
- Sodium cyanoborohydride
-
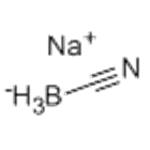
- 2025-12-14
- CAS:25895-60-7
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sodium cyanoborohydride
-

- $41.00 / 25g
- 2025-12-12
- CAS:25895-60-7
- Min. Order: 25g
- Purity: 0.95
- Supply Ability: 100kg
- Sodium cyanoborohydride
-

- $10.00 / 1KG
- 2025-12-11
- CAS:25895-60-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt