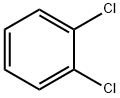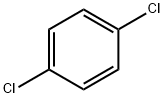
1,4-Dichlorobenzene synthesis
- Product Name:1,4-Dichlorobenzene
- CAS Number:106-46-7
- Molecular formula:C6H4Cl2
- Molecular Weight:147
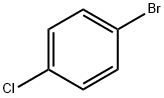
106-39-8
475 suppliers
$14.00/5g

106-46-7
561 suppliers
$10.00/5g
Yield:106-46-7 95%
Reaction Conditions:
with trans bis(glycinato)copper(II) monohydrate;anhydrous tetramethylammonium chloride in ethanol at 100; for 24 h;Schlenk technique;Inert atmosphere;Finkelstein Reaction;
Steps:
General procedure for aryl and heteroaryl bromide-chloride exchange reaction
General procedure: A Schlenk tube was charged with catalyst 1 (10 mol %), aryl (or heteroaryl) bromide (1.0 mmol), tetramethylammonium chloride (Me4NCl) (2.0 mmol), and EtOH (2.0 mL) under nitrogen atmosphere. The Schlenk tube was sealed with a Teflon valve, and then the reaction mixture was stirred at 100 °C for a period as mentioned in Table 2 (the reaction progress was monitored by GC analysis). After completion of the reaction, the solvent was removed under reduced pressure. The residue obtained was purified via silica gel chromatography (eluent: petroleum ether/ethyl acetate = 10/1) to afford aryl chlorides. The yield of the products was determined by high resolution GC-MS analysis and the identity of the products was confirmed by comparing their physical and spectral data with the known compounds.
References:
Verma, Sanny;Saran, Sandeep;Jain, Suman L. [Applied Catalysis A: General,2014,vol. 472,p. 178 - 183]
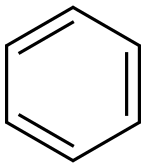
71-43-2
721 suppliers
$11.19/25ML

106-46-7
561 suppliers
$10.00/5g
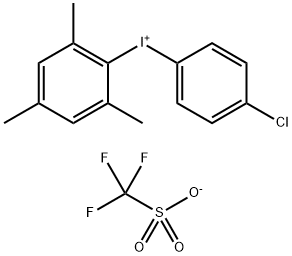
1204518-00-2
9 suppliers
inquiry

106-46-7
561 suppliers
$10.00/5g
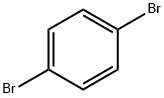
106-37-6
479 suppliers
$9.00/5g

106-46-7
561 suppliers
$10.00/5g