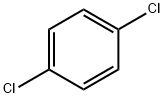
1,4-Dichlorobenzene
- CAS No.
- 106-46-7
- Chemical Name:
- 1,4-Dichlorobenzene
- Synonyms
- Dichlorobenzene;p-Dichlorobenzene;PARA;PDB;PDCB;1,4-dcb;DICHLOROBENZENES;PARA DICHLOROBENZENE;p-Diclorobenzene;1,4-Dichlorbenzol
- CBNumber:
- CB9329690
- Molecular Formula:
- C6H4Cl2
- Molecular Weight:
- 147
- MDL Number:
- MFCD00000604
- MOL File:
- 106-46-7.mol
- MSDS File:
- SDS
| Melting point | 52-54 °C (lit.) |
|---|---|
| Boiling point | 173 °C (lit.) |
| Density | 1.241 g/mL at 25 °C (lit.) |
| vapor density | 5.07 (vs air) |
| vapor pressure | 1.03 mm Hg ( 25 °C) |
| refractive index | 1.5434 |
| Flash point | 150 °F |
| storage temp. | 2-8°C |
| solubility | 0.08g/l |
| form | Crystals |
| color | White |
| Specific Gravity | 1.2417 |
| PH | 7 (0.06g/l, H2O, 20℃) |
| Odor | Aromatic. |
| explosive limit | 1.7-5.9%(V) |
| Water Solubility | insoluble |
| Merck | 14,3057 |
| BRN | 1680023 |
| Henry's Law Constant | 1.88 at 25 °C (continuous flow sparger, Sproule et al., 1991) |
| Exposure limits | TLV-TWA 75 ppm (~450 mg/m3) (MSHA, OSHA, and NIOSH); IDLH 1000 ppm (NIOSH). |
| Dielectric constant | 2.8(53℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, aluminium and its alloys, some plastics. |
| EPA Primary Drinking Water Standard | MCL:0.075,MCLG:0.075 |
| LogP | 3.37 at 25℃ and pH7 |
| Indirect Additives used in Food Contact Substances | P-DICHLOROBENZENE |
| CAS DataBase Reference | 106-46-7(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | D149TYB5MK |
| Proposition 65 List | p-Dichlorobenzene |
| NIST Chemistry Reference | Benzene, 1,4-dichloro-(106-46-7) |
| IARC | 2B (Vol. Sup 7, 73) 1999 |
| EPA Substance Registry System | p-Dichlorobenzene (106-46-7) |
| Pesticides Freedom of Information Act (FOIA) | Paradichlorobenzene |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H351-H410 | |||||||||
| Precautionary statements | P202-P264-P273-P280-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 36-40-50/53-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 36/37-46-60-61-45-16-7 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CZ4550000 | |||||||||
| Autoignition Temperature | >500 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29036100 | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 3863, 3790 orally; >6000, >6000 dermally (Gaines, Linder) | |||||||||
| IDLA | 150 ppm | |||||||||
| NFPA 704 |
|
1,4-Dichlorobenzene price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.03226 | 1,4-Dichlorobenzene for synthesis | 106-46-7 | 100g | $33.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03226 | 1,4-Dichlorobenzene for synthesis | 106-46-7 | 2.5kg | $98.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40025 | 1,4-Dichlorobenzene solution certified reference material, 5000?μg/mL in methanol | 106-46-7 | 1mL | $62.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03226 | 1,4-Dichlorobenzene for synthesis | 106-46-7 | 1kg | $52.6 | 2023-06-20 | Buy |
| Sigma-Aldrich | 35775 | 1,4-Dichlorobenzene PESTANAL | 106-46-7 | 1g | $21.3 | 2022-05-15 | Buy |
1,4-Dichlorobenzene Chemical Properties,Uses,Production
Description
1,4-Dichlorobenzene (also known as p-dichlorobenzene) is a chlorinated aromatic compound with a distinctive aromatic odor that is very strong at high concentrations. It is a white or colorless crystal at room temperature (Akron 2009, HSDB 2009). 1,4-Dichlorobenzene is practically insoluble in water; soluble in chloroform, carbon disulfide, benzene, and ether; and very soluble in ethanol and acetone. 1,4-Dichlorobenzene is noncorrosive, volatile, and combustible, and it is flammable when exposed to heat, flame, or oxidizers. When it is heated to decomposition, toxic gases and vapors (such as hydrochloric acid and carbon monoxide) are released (HSDB 2009). It is stable at room temperature under normal handling and storage in closed containers (Akron 2009).
1,4-Dichlorobenzene is the primary ingredient in mothballs and deodorant cakes placed in toilet bowls, urinals, and animal holding facilities. People may also use it to control lice and mites in and around birdcages. 1,4-Dichlorobenzene is used as an insecticide on fruit and is used to control mold and mildew growth on tobacco seeds, leather, and certain fabrics. It is also approved for controlling wax moths in empty, stored beehives.
Chemical Properties
There are three isomeric forms of dichlorobenzene (DCB): m-DCB is a flammable liquid and vapor.
Physical properties
Colorless to white crystals with a penetrating, sweet, mothball or almond-like odor. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 18 and 4.5 μg/L, respectively. Similarly, at 25 °C, the average taste threshold concentration and the lowest concentration at which a taste was detected were 32 and 11 μg/L, respectively (Young et al., 1996). A detection odor threshold concentration of 73 μg/m3 (121 ppbv) was reported by Punter (1983).
Uses
1,4-Dichlorobenzene is used to make mothballs and solid deodorant blocks for garbage cans and restrooms. It is also used to control odors in places where animals are held. It has been used as an insecticide on fruit, and to control mold and mildew on tobacco seeds, leather and some fabrics. 1,4-Dichlorobenzene is sent into the air by plants that make or use it and a small amount is released to soil and water. This chemical can also be detected in indoor air where products containing 1,4-Dichlorobenzene are used.
Application
1,4-Dichlorobenzene is used as a fumigantand as an insecticide. For domestic use against clothes moths; as space deodorant in room deodorizers, toilet bowl blocks and diaper pail deodorizers. Intermediate in production of plastics for electronic components.
Preparation
1,4-Dichlorobenzene was first produced commercially in the United States in 1915 (IARC 1982). It is produced by reacting liquid benzene with gaseous chlorine in the presence of a catalyst at moderate temperature and atmospheric pressure. 1,4-Dichlorobenzene is used mainly as a fumigant for the control of moths, molds, and mildews, and as a space deodorant for toilets and refuse containers.
Definition
ChEBI: 1,4-dichlorobenzene is a dichlorobenzene carrying chloro groups at positions 1 and 4. It has a role as an insecticide.
Synthesis Reference(s)
Chemistry Letters, 8, p. 939, 1979
The Journal of Organic Chemistry, 48, p. 250, 1983 DOI: 10.1021/jo00150a020
Tetrahedron Letters, 23, p. 371, 1982 DOI: 10.1016/S0040-4039(00)86833-1
General Description
A white colored liquid with the odor of moth balls. Denser than water and insoluble in water. Flash point below 200°F. Used as a moth repellent, to make other chemicals, as a fumigant, and for many other uses.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,4-Dichlorobenzene is incompatible with oxidizing agents. 1,4-Dichlorobenzene is also incompatible with aluminum and its alloys. 1,4-Dichlorobenzene liquefies when mixed with camphor, phenol and salol. 1,4-Dichlorobenzene will attack some forms of plastics, rubber and coatings. .
Health Hazard
Toxic symptoms are headache, weakness,dizziness, nausea, vomiting, diarrhea, loss ofweight, and injury to liver and kidney. Thesesymptoms occur from repeated inhalationof high concentrations of vapors or fromingestion. The vapors are an irritant to theeyes, throat, and skin. Chronic exposure maycause jaundice and cirrhosis. The oral LD50value in mice is in the range 3000 mg/kg.The fatal oral dose in humans is estimated tobe 40–50 g. Carcinogenic studies on animalshave not produced adequate evidence of anycancer-causing action.
Fire Hazard
Special Hazards of Combustion Products: Vapors are irritating. Toxic chlorine, hydrogen chloride, and phosgene gases may be generated in fires.
Trade name
DowTHERM®; EVOLA; PARACIDE®; PARA CRYSTALS®; PARADI®; PARADOW®; PARAMOTH®; PARANUGGETS®; PARAZENE®; PERSIA-PERAZOL®; SANTOCHLOR®; Mixed isomers: DILATIN DBI®; MOTTENSCHUTZMITTEL EVAU P®; MOTT-EX®; TOTAMOTT®
Safety Profile
There is limited evidence that 1,4-dichlorobenzene can damage a developing fetus. Exposure can damage the lungs, liver, kidneys, and blood cells, causing anemia; it can also cause swelling of the eyes, hands, and feet. It can damage the nervous system, causing weakness, trembling, and numbness in the arms and legs. It may cause a skin allergy, which when developed can cause itching and a skin rash. Higher levels of the chemical in air, such as the levels that are sometimes associated with industrial exposure, can cause headaches, nausea, clumsiness, slurred speech, and dizziness. Levels that would result in death would be associated with an odor so intense that it would be very unpleasant, if not intolerable, and would serve as a danger warning. In industrial situations, workers exposed to 1,4-dichlorobenzene at high levels are usually directed to wear respirators. Workers involved in the production of the chemical may be exposed to concentrations significantly higher than those encountered by the general population. High exposure levels may result from some consumer products of moth repellents and room deodorizers. Approximately 95% of the environmental release of 1,4-dichlorobenzene occurs during its use, rather than during its manufacture or processing.
Potential Exposure
The major uses of o-DCB are as a process solvent in the manufacturing of toluene diisocyanate and as an intermediate in the synthesis of dyestuffs, herbicides, and degreasers. p-Dichlorbenzene is used primarily as a moth repellant, a mildew control agent; space deodorant; and in insecticides, which accounts for 90% of the total production of this isomer. Information is not available concerning the production and use of m-DCB. However, it may occur as a contaminant of o-or p-DCB formulations. Both o-and p-isomers are produced almost entirely as by-products during the production of monochlorobenzene
Carcinogenicity
1,4-Dichlorobenzene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Metabolic pathway
1,4-Dichlorobenzene undergoes degradation by the Xanthobacter flavus 14p1 isolated from river sludge by selective enrichment with 1,4-dichlorobenzene, resulting in the degradation products 3,6-dichloro-cis- 1,2-dihydroxycyclohexa-3,5-diene and 3,6- dichlorocatechol. 2,5-Dichloromuconic acid and 2- chloromaleylacetic acid, as well as the decarboxylation product 2-chloroacetoacrylic acid, are identified after enzymatic conversion of 3,6-dichlorocatechol.
Shipping
m-DCB: UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. United States DOT Regulated Marine Pollutant. UN3077 Environmentally hazardous substances, solis, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical NameRequired. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
o-Dichlorobenzene is a common impurity. The p-isomer has been purified by steam distillation, crystallisation from EtOH or boiling MeOH, air-dried and dried in the dark under vacuum. It has also been purified by zone refining. [Beilstein 5 IV 658.]
Incompatibilities
For o-DCB and m-DCB: acid fumes, chlorides, strong oxidizers; hot aluminum, or aluminum alloys. For p-DCB: Strong oxidizers; although, incompatibilities for this chemical may also include other materials listed for o-DCB.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
1,4-Dichlorobenzene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BLiT (Hefei)Chemical Co.,Ltd | +86-551-62622640 | sales@blitchem.com | China | 293 | 58 |
| HUAIAN DCHEN CHEM CO LTD | +86-57-80696996 +86-19816093969 | dchenchem@gmail.com | China | 16 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12465 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5895 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20291 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1098 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Speranza Chemical Co., Ltd. | +86-86-075521030354 +8618688942810 | sophieliu@speranzachem.com | China | 801 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
Related articles
- 1,4-Dichlorobenzene: Uses and Hazard
- 1,4-Dichlorobenzene is a colorless to white flammable crystalline solid at room temperature that slowly evaporates when expose....
- Jun 3,2024
View Lastest Price from 1,4-Dichlorobenzene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | 1,4-dichlorobenzene
106-46-7
|
US $5.00 / KG | 1KG | 99% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-08-15 | 1,4-dichlorobenzene
106-46-7
|
US $0.00 / kg | 1kg | 0.99 | 1000kg | Hebei Yanxi Chemical Co., Ltd. | |
 |
2024-08-14 | 1,4-Dichlorobenzene
106-46-7
|
US $0.00 / kg | 1kg | 99% | 50000KG/month | Hebei Mojin Biotechnology Co.,Ltd |
-

- 1,4-dichlorobenzene
106-46-7
- US $5.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- 1,4-dichlorobenzene
106-46-7
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- 1,4-Dichlorobenzene
106-46-7
- US $0.00 / kg
- 99%
- Hebei Mojin Biotechnology Co.,Ltd
106-46-7(1,4-Dichlorobenzene)Related Search:
1of4