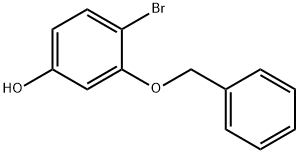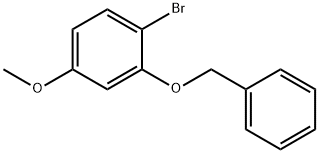
1-BROMO-4-METHOXY-2-PHENYLMETHOXYBENZENE synthesis
- Product Name:1-BROMO-4-METHOXY-2-PHENYLMETHOXYBENZENE
- CAS Number:150356-67-5
- Molecular formula:C14H13BrO2
- Molecular Weight:293.16
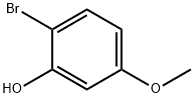
63604-94-4
153 suppliers
$7.00/250mg

7732-18-5
508 suppliers
$13.50/100ML
584-08-7
1254 suppliers
$5.00/100g

100-44-7
659 suppliers
$13.50/250G

75-05-8
1050 suppliers
$10.00/10g

150356-67-5
30 suppliers
$134.00/1g
Yield:150356-67-5 99%
Reaction Conditions:
with Ki;nitrogen in ethyl acetate;
Steps:
4.i [4-Methoxy-2(phenylmethoxy)phenyl]boronic acid (a compound of Formula (u))
(i) 1-Bromo-4-methoxy-2-(phenylmethoxy)benzene (a compound of Formula (aa)) A 12 L four-necked round-bottomed flask equipped with mechanical stirrer, thermometer and reflux condenser with nitrogen inlet was charged with 2-bromo-5-methoxyphenol (615 g, 2.98 mol), CH3 CN (6 L), K2 CO3 (671 g, 4.77 mol), benzyl chloride (363 mL, 3.14 mol), and KI (6 g, 0.036 mol). The mixture was heated to reflux for 3 h, cooled to room temperature, and H2 O (3 L) added to form a biphasic mixture. The aqueous layer was separated, CH3 CN (3 L) removed in vacuo and EtOAc (4 L) added to extract the product. After a wash with H2 O (3 L) and 10% NaCl solution (1.8 L), the organic layer was concentrated to dryness to afford 1-bromo-4-methoxy-2-(phenylmethoxy)benzene as a light yellow oil (895.5 g, 99%): bp 110° C. (2.5 mm Hg); 1 H NMR δ 7.55-7.25 (m, 6 H), 6.55 (d, J=2.7 Hz, 1 H), 6.35 (dd, J=8.5 Hz, 1.5 Hz, 1 H), 5.1 (s, 2 H), 3.75 (s, 3 H).
References:
US6143907,2000,A
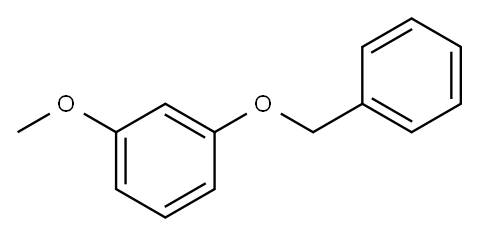
21144-16-1
5 suppliers
inquiry
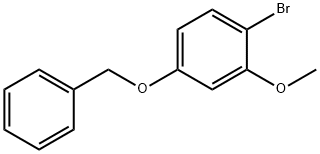
171768-67-5
48 suppliers
$158.00/1g

150356-67-5
30 suppliers
$134.00/1g

63604-94-4
153 suppliers
$7.00/250mg

100-39-0
432 suppliers
$10.00/10g

150356-67-5
30 suppliers
$134.00/1g
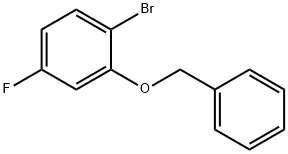
202857-88-3
50 suppliers
$67.00/500mg

67-56-1
790 suppliers
$9.00/25ml

150356-67-5
30 suppliers
$134.00/1g