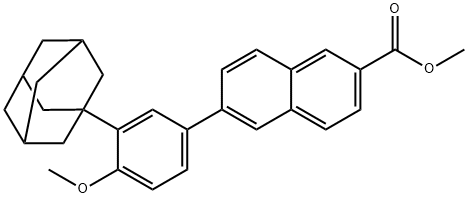
Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate synthesis
- Product Name:Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate
- CAS Number:106685-41-0
- Molecular formula:C29H30O3
- Molecular Weight:426.55
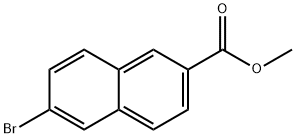
33626-98-1
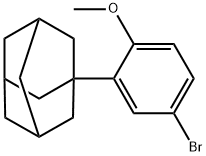
104224-63-7
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
General procedure for the preparation of methyl 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthalenecarboxylate (V): magnesium scraps (1.26 g, 51.85 mmol) were mixed with THF (10 mL) under nitrogen protection and stirred at room temperature. 2-(1-adamantyl)-4-bromoanisole (IV) (1.4 g, 4.36 mmol) and 1,2-dibromoethane (0.56 mL) were added, and the reaction mixture was subsequently heated to 40 °C to initiate the reaction. Then a solution of 2-(1-adamantyl)-4-bromoanisole (12.6 g, 39.25 mmol) in THF (40 mL) was slowly added dropwise under reflux conditions for 30 min. A solution of purified zinc chloride (8.4 g, 61 mmol) in THF (30 mL) was added slowly dropwise at reflux temperature over 15 minutes. After the reaction mixture was refluxed for 1 h, methyl 6-bromo-2-naphthalenecarboxylate (8.0 g, 30 mmol) was added and stirred for 10 min, followed by the addition of NiCl2ZDPPE catalyst (0.21 g). The mixture was continued to be stirred at the same temperature for 2 h. The residue was subsequently concentrated. The residue was treated with dichloromethane (100 mL) and 1N HCl (100 mL), and the dichloromethane layer was washed sequentially with 10% EDTA disodium salt solution and water, dried with anhydrous sodium sulfate, and distilled to give the crude product. The crude product was stirred in ethyl acetate (140 mL) at 70 °C for 1 h, cooled to 15 °C and kept for 1 h. The solid was collected by filtration and dried. [Yield: 9.45 g, 50% yield].
106685-40-9
625 suppliers
$6.00/50mg

18107-18-1
211 suppliers
$33.00/5g
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
167 suppliers
$21.00/100mg
Yield:106685-41-0 79%
Reaction Conditions:
in tetrahydrofuran;methanol at 0 - 20; for 1 h;
Steps:
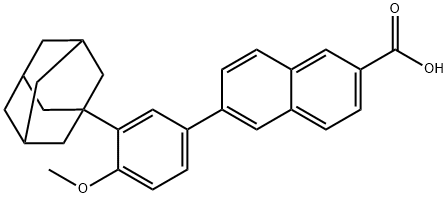
1.5 Methyl 6-(3-(adamantan-l-yl)-4-methoxyphenyl)-2-naphthoate (5).
To a solution of adapalene (50 mg, 0.121 mmol) in 4: 1 THF/MeOH (0.4 mL) at 0 °C was added TMSCH2N2 (0.15 mL, 0.290 mmol) and the reaction was warmed to room temperature over 1 hour. The reaction mixture was concentrated, IN HCl was added, and was extracted with EtOAc 3x. The combined organic layers were washed with water and brine, dried over Na2S04, filtered, and concentrated; yielding the title compound as a white solid (41 mg, 79% yield). NMR (500 MHz, CDC13) δ 8.61 (s, 1H), 8.07 (dd, J = 8.6, 1.7 Hz, 1H), 8.03 - 7.96 (m, 2H), 7.92 (d, J = 8.6 Hz, 1H), 7.80 (dd, J = 8.5, 1.8 Hz, 1H), 7.60 (d, J = 2.4 Hz, 1H), 7.55 (dd, J = 8.4, 2.4 Hz, 1H), 7.00 (d, J = 8.5 Hz, 1H), 3.99 (s, 3H), 3.91 (s, 3H), 2.19 (s, 6H), 2.10 (s, 3H), 1.80 (s, 6H); 13C NMR (125 MHz, CDC13) δ 167.44, 159.03, 141.51, 139.11, 136.06, 132.67, 131.35, 130.95, 129.82, 128.33, 127.02, 126.59, 126.09, 125.84, 125.68, 124.84, 112.21, 55.27, 52.31, 40.72, 37.25, 29.23; HRMS Accurate mass (ES+): Found 427.2268, C29H3i03 (M+H+) requires 427.2273.
References:
AUSUBEL, Frederick M.;KIM, Wooseong;MYLONAKIS, Eleftherios;WUEST, William M. WO2018/213609, 2018, A1 Location in patent:Page/Page column 93

33626-98-1
321 suppliers
$16.80/1g

104224-63-7
302 suppliers
$16.00/5g
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
167 suppliers
$21.00/100mg

33626-98-1
321 suppliers
$16.80/1g

104224-63-7
302 suppliers
$16.00/5g
932033-57-3
68 suppliers
$130.00/50mg
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
167 suppliers
$21.00/100mg
![2-Naphthalenecarboxylic acid, 6-(4-hydroxy-3-tricyclo[3.3.1.13,7]dec-1-ylphenyl)-, methyl ester](/CAS/20210305/GIF/106685-43-2.gif)
106685-43-2
1 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
167 suppliers
$21.00/100mg
459423-32-6
98 suppliers
$50.00/25mg
![2-Naphthalenecarboxylic acid, 6-[(4-methylphenyl)sulfonyl]-, methyl ester](/CAS/20210305/GIF/366799-67-9.gif)
366799-67-9
0 suppliers
inquiry
![Mehtyl 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoate](/CAS/GIF/106685-41-0.gif)
106685-41-0
167 suppliers
$21.00/100mg