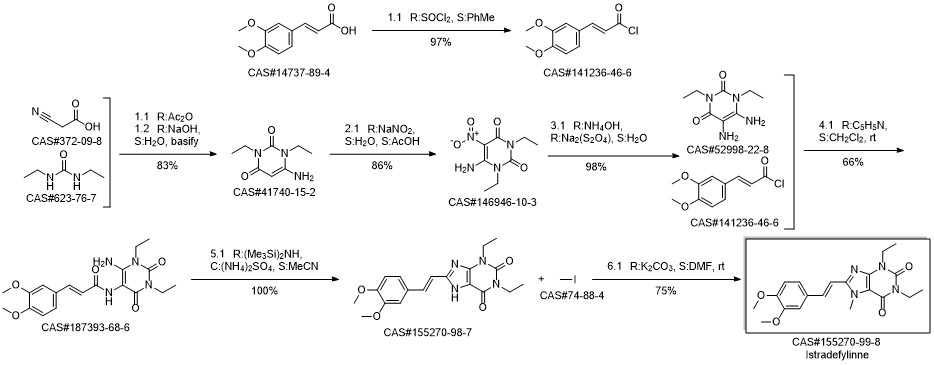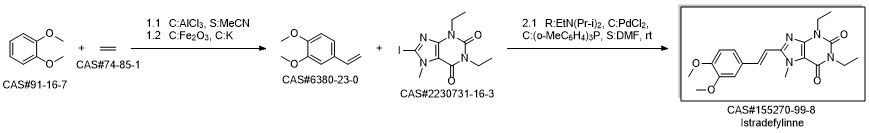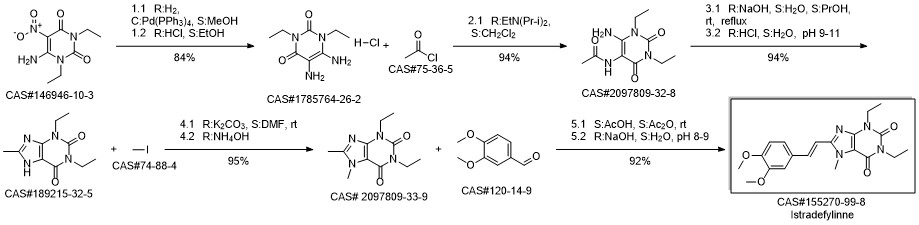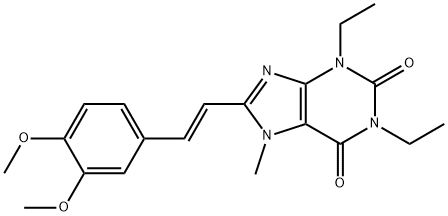
8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione synthesis
- Product Name:8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione
- CAS Number:155270-99-8
- Molecular formula:C20H24N4O4
- Molecular Weight:384.43
Reference: Li, Fan; Hou, Xingpu; Li, Lin; Lu, Tao; Du, Yumin. Synthesis of antiparkinsonian agent istradefylline. Zhongguo Yiyao Gongye Zazhi. Volume 41. Issue 4. Pages 241-243. Journal. (2010).
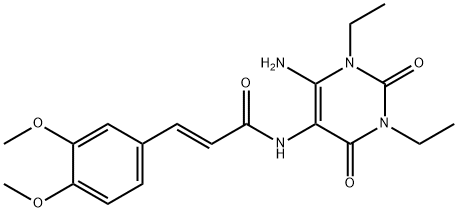
![8-[(1E)-2-(3,4-Dimethoxyphenyl)ethenyl]-1,3-diethyl-3,9-dihydro-1H-purine-2,6-dione](/CAS2/GIF/155270-98-7.gif)
155270-98-7
37 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](/CAS/GIF/155270-99-8.gif)
155270-99-8
223 suppliers
$50.00/250 mg
Yield:155270-99-8 99%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 50; for 5 h;
Steps:
1 Preparation Example 1: Preparation of crude istradefylline
Under the mechanical stirring, into 3L reaction flask, add intermediate II 50g (0.135 mol), potassium carbonate 28g (0.203 mol), N,N-dimethylformamide 550 ml and iodomethane 15 ml (0.241 mol), heated to 50 °C, stirring reaction 5h, cooled to 0 °C, add purified water to the reaction solution in 550 ml. Filtering, cake purified water 1.1L after washing, 45 °C lower vacuum drying, to obtain 51.3g pale yellow solid, yield 99%, HPLC purity 98.6%, used in the following istradefylline A crystal preparation.
References:
CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd.;Gong, Denghuang;Wang, Jie;Ge, Jinghua;Yang, Jie;Sun, Wenjiao;Yang, Min;Yang, Chunqiao;Ma, Yuxiu CN106279169, 2017, A Location in patent:Paragraph 0044; 0045; 0046

616-38-6
722 suppliers
$5.00/5 g
![8-[(1E)-2-(3,4-Dimethoxyphenyl)ethenyl]-1,3-diethyl-3,9-dihydro-1H-purine-2,6-dione](/CAS2/GIF/155270-98-7.gif)
155270-98-7
37 suppliers
inquiry
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](/CAS/GIF/155270-99-8.gif)
155270-99-8
223 suppliers
$50.00/250 mg

77-78-1
319 suppliers
$19.69/1ml
![8-[(1E)-2-(3,4-Dimethoxyphenyl)ethenyl]-1,3-diethyl-3,9-dihydro-1H-purine-2,6-dione](/CAS2/GIF/155270-98-7.gif)
155270-98-7
37 suppliers
inquiry
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](/CAS/GIF/155270-99-8.gif)
155270-99-8
223 suppliers
$50.00/250 mg

616-38-6
722 suppliers
$5.00/5 g
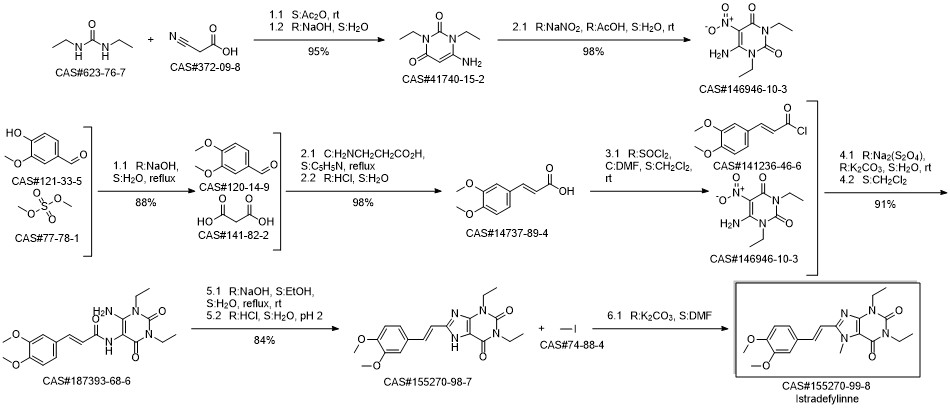
187393-68-6
26 suppliers
inquiry
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](/CAS/GIF/155270-99-8.gif)
155270-99-8
223 suppliers
$50.00/250 mg
31617-39-7
4 suppliers
inquiry
6380-23-0
92 suppliers
$30.00/1g
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](/CAS/GIF/155270-99-8.gif)
155270-99-8
223 suppliers
$50.00/250 mg