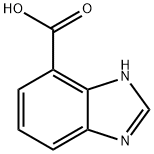
1H-Benzimidazole-4-carboxylic acid synthesis
- Product Name:1H-Benzimidazole-4-carboxylic acid
- CAS Number:46006-36-4
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15

64-18-6
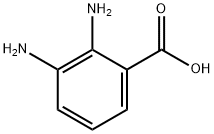
603-81-6

46006-36-4
To a 4M hydrochloric acid (20 mL) solution of formic acid (0.75 mL, 20.0 mmol) was added 2,3-diaminobenzoic acid (1.00 g, 6.6 mmol). The reaction mixture was heated at reflux temperature for 2 h and subsequently cooled to room temperature. The resulting pink precipitate was separated by filtration and dissolved in hot methanol. Decolorization was carried out by adding activated carbon and stirring for 10 min. The suspension was filtered and the solvent was evaporated under reduced pressure to afford benzimidazole-7-carboxylic acid (0.542 g, 51% yield) as white crystals with a melting point of 266-268 °C. The structure of the product was analyzed by 1H NMR (300 MHz, DMSO-d6) δ 9.64 (s, 1H), 8.11 (dd, J = 8.2, 0.9 Hz, 1H), 8.07 (dd, J = 7.6, 0.9 Hz, 1H), 7.65 (dd, J = 8.2, 7.6 Hz, 1H) and 13C NMR (75 MHz, DMSO-d6 ) δ 265.2, 142.0, 132.3, 129.3, 127.6, 125.6, 119.7, 117.6 confirmed.
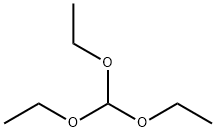
122-51-0
514 suppliers
$10.00/5ml

603-81-6
200 suppliers
$7.00/250mg

46006-36-4
150 suppliers
$19.00/250mg
Yield: 98%
Reaction Conditions:
at 130;
Steps:
5.1.92.A
A. lH-Benzo[d]imidazole-4-carboxyIic acid.; 2,3-Diaminobenzoic acid (1.0 g, 6.57 mmol) was suspended in triethylorthoformate (20 mL) and heated to 130 0C overnight. Diethyl ether (100 mL) was then added and the resulting precipitate filtered to give a white solid (1.04 g, 6.41 mmol, 98%). 1H NMR (400 MHz, DMSO-rf6) δ 8.22 (s, IH), 7.91 (dd, J = S.0, 1.2, IH), 7.82 (dd, J = 8.0, 1.2, IH), 7.29 (dd, J = 8.0, 7.6, IH); MS (ESI) m/z 163.0 [M+ 1]+.
References:
SIGNAL PHARMACEUTICALS, LLC WO2008/51494, 2008, A1 Location in patent:Page/Page column 132

64-18-6
1132 suppliers
$22.12/250ML

603-81-6
200 suppliers
$7.00/250mg

46006-36-4
150 suppliers
$19.00/250mg

64-18-6
1132 suppliers
$22.12/250ML

46006-36-4
150 suppliers
$19.00/250mg

603-81-6
200 suppliers
$7.00/250mg
208772-23-0
55 suppliers
$45.00/10mg

46006-36-4
150 suppliers
$19.00/250mg
606-18-8
336 suppliers
$9.00/1g

46006-36-4
150 suppliers
$19.00/250mg