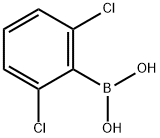
2,6-DICHLOROPHENYLBORONIC ACID synthesis
- Product Name:2,6-DICHLOROPHENYLBORONIC ACID
- CAS Number:73852-17-2
- Molecular formula:C6H5BCl2O2
- Molecular Weight:190.82
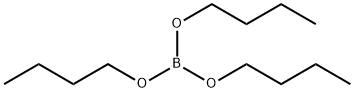
688-74-4
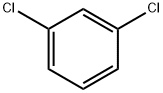
541-73-1

73852-17-2
(1) Lithiation reaction: Add 50 g of 1,3-dichlorobenzene to a 1000 mL three-necked flask under nitrogen atmosphere and cool to -80 to -60°C. Slowly add 176 mL of n-butyl lithium (n-BuLi) dropwise. Slowly add 176 mL of n-Butyl lithium (n-BuLi) dropwise and keep the reaction temperature between -80 and -50℃. After the dropwise addition, the reaction was continued for 2 hours. (2) Lithium-hydrogen exchange reaction: 117 g of tributyl borate (B(OBu)3) was added dropwise to the phenyl lithium solution prepared above, and the reaction temperature was maintained at a holding temperature after the dropwise addition was completed. At the end of the reaction, 10% hydrochloric acid solution was added to the system for acidification, stirred and left to stratify. The organic layers were separated, combined and the solvent was removed, and treated with hydrated succinamide to obtain 51.7 g of crude 2,6-dichlorophenylboronic acid in 80% yield. (3) Purification step: 51.7 g of crude 2,6-dichlorophenylboronic acid was placed in a 1 L three-necked flask and dissolved by adding 200 mL of methyl tert-butyl ether (MTBE). After removing the solvent, cooled and crystallized, centrifuged to obtain 48.4 g of pure 2,6-dichlorophenylboronic acid in 75% yield.

688-74-4
337 suppliers
$10.40/25mL

541-73-1
410 suppliers
$19.00/25G

73852-17-2
238 suppliers
$6.00/1g
Yield: 75%
Reaction Conditions:
Stage #1:1,3-Dichlorobenzene with n-butyllithium at -80 - -50; for 2 h;Inert atmosphere;
Stage #2:boric acid tributyl esterInert atmosphere;Temperature;
Steps:
1 Example 1
(1) Lithiation: under the protection of nitrogen,First in a 1000mL three-necked flask,Add 50g of 1,3-dichlorobenzene and cool to -80~-60°C.Then add 176 mL of n-butyl lithium dropwise.The dropping process is maintained at -80 to -50 ° C.After the completion of the dropwise addition, the reaction was kept for 2 hours.(2) Lithium-hydrogen exchange:117 g of tributyl borate liquid was added dropwise to the solution of the above-obtained phenyl lithium,After the addition is completed, the heat retention reaction is carried out.After the reaction, the reaction system was stirred by adding hydrochloric acid having a mass concentration of 10%.Then let go of the layering,Extracted,Combine the organic layers,Desolvent,Hydrating succinol to obtain 51.7 g of crude 4-phenoxybenzeneboronic acid in a yield of 80%;(3) Purification:51.7 g of crude 4-phenoxyphenylboronic acid was added to a 1 L three-necked flask.Add 200 mL of methyl tert-butyl ether to dissolve,Desolvent,Cooling, crystallization, and centrifugation gave 48.4 g of 4-phenoxybenzeneboronic acid product in a yield of 75%.
References:
Zhuhai Aobokai Bio-pharmaceutical Co., Ltd.;Zhou Yunfen;Yang Chunpeng;Zeng Mengjing CN108084217, 2018, A Location in patent:Paragraph 0006; 0017-0020; 0022-0024; 0026-0028; 0030-0032
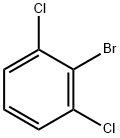
19393-92-1
191 suppliers
$10.00/5g
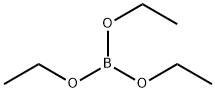
150-46-9
200 suppliers
$12.00/25mL

73852-17-2
238 suppliers
$6.00/1g

108-86-1
523 suppliers
$10.00/5g

688-74-4
337 suppliers
$10.40/25mL

7732-18-5
507 suppliers
$12.69/100ml

73852-17-2
238 suppliers
$6.00/1g

19393-92-1
191 suppliers
$10.00/5g

688-74-4
337 suppliers
$10.40/25mL

73852-17-2
238 suppliers
$6.00/1g

19393-92-1
191 suppliers
$10.00/5g

121-43-7
381 suppliers
$13.00/25mL

73852-17-2
238 suppliers
$6.00/1g