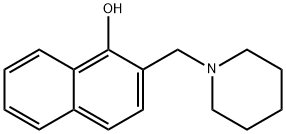
2-(PIPERIDINOMETHYL)-1-NAPHTHOL synthesis
- Product Name:2-(PIPERIDINOMETHYL)-1-NAPHTHOL
- CAS Number:6638-91-1
- Molecular formula:C16H19NO
- Molecular Weight:241.33
110-89-4
3 suppliers
$15.96/100ML

50-00-0
838 suppliers
$10.00/25g
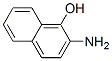
606-41-7
19 suppliers
inquiry

6638-91-1
17 suppliers
inquiry
Yield:6638-91-1 92%
Reaction Conditions:
with gallium;water at 40; for 1 h;
Steps:
10
A process for the preparation of an aminonaphthol compound is carried out by adding the catalyst gallium powder, paraformaldehyde, piperidine and α-naphthol successively to water at a temperature of 50 ° C, The whole reaction process is tracked by thin layer chromatography, that is, every 5 minutes sampling, with the capillary in the silica gel plate were added to the reaction solution point, raw material, α-naphthol point, three points in the same line , And then the silica gel plate was placed in a vial containing a mixture of petroleum ether and ethyl acetate in a volume ratio (mL / mL) of 30: 1.After the completion of the board, and then the silicone plate on the UV lamp or iodine bottle in the observation, if the reaction solution is not with the raw material α-naphthol flush point, then the reaction is complete, then the reaction solution.The reaction solution was extracted three times with the same amount of ethyl acetate as the water, and the organic phase was combined to obtain an extract.The anhydrous magnesium sulfate was added to the extract to dry it, and the filtrate was filtered to obtain a filtrate. The solvent was distilled off under reduced pressure at 40 ° C using a RE-52AA rotary evaporator produced by Haoyalong Biochemical Co., Ltd. to obtain a concentrate; And then add 2 to 3 times the concentration of concentrated liquid in the concentration of silica gel for mixing, the sample into the column, adding volume ratio (mL / mL) of 30: 1 mixture of petroleum ether and ethyl acetate And the eluate was collected in a rotary evaporator and concentrated at 40 ° C to obtain the aminonaphthol compound of the product II. The product was of the formula (wherein R1= H, R2= - (CH2)5-), Wherein the mole ratio of gallium powder to α-naphthol is 1: 10, the molar ratio of α-naphthol to paraformaldehyde is 1: 1.1, the molar ratio of α-naphthol to piperidine is 1: 1, The molar ratio of naphthol to water is 1: 1000.The reaction time was 1 h and the yield was 92%.
References:
CN103664826,2016,B Location in patent:Paragraph 0055; 0056; 0057; 0058; 0059; 0060

110-89-4
3 suppliers
$15.96/100ML

50-00-0
838 suppliers
$10.00/25g
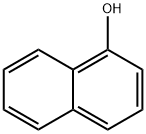
90-15-3
694 suppliers
$9.00/5g

6638-91-1
17 suppliers
inquiry

110-89-4
3 suppliers
$15.96/100ML

50-00-0
838 suppliers
$10.00/25g

64-17-5
693 suppliers
$10.00/50g

90-15-3
694 suppliers
$9.00/5g

6638-91-1
17 suppliers
inquiry