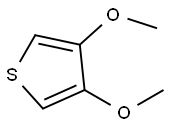
3,4-Dimethoxythiophene synthesis
- Product Name:3,4-Dimethoxythiophene
- CAS Number:51792-34-8
- Molecular formula:C6H8O2S
- Molecular Weight:144.19
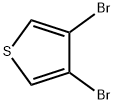
3141-26-2

124-41-4

51792-34-8
Under argon protection, 21 g of sodium methanolate and 72 g of methanol were added to a 100 mL four-necked flask (the initial concentration of sodium methanolate in methanol was 22.6 wt%) and stirred until complete dissolution. Subsequently, 0.83 g of cuprous bromide was added as a catalyst and 15 g of 3,4-dibromothiophene was slowly added dropwise, and the reaction solution gradually changed from colorless to black transparent. After the dropwise addition was completed, 50 g of methanol was removed by distillation (at this time the concentration of sodium methanolate in the remaining methanol rose to 48.8 wt%). The reaction mixture was heated to 97 °C for reflux reaction. The levels of 3,4-dibromothiophene and 3-bromo-4-methoxythiophene were monitored by gas chromatography and were below the detection limit after 5 h of reaction. After completion of the reaction, water was added to the mixture, filtered and the crude product was extracted with toluene. The toluene layer was sequentially washed with water and dried over magnesium sulfate. After filtration to remove the desiccant, the toluene layer was concentrated by rotary evaporation and finally vacuum distilled to give 7.28 g of 3,4-dimethoxythiophene in 81.5% yield. The product was analyzed by gas chromatography (Agilent 6890N network GC, FID detector) and the purity was 98.01%.

3141-26-2
311 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g

51792-34-8
321 suppliers
$6.00/1g
Yield:51792-34-8 81.5%
Reaction Conditions:
with copper(I) bromide in methanol at 70 - 97;Inert atmosphere;Temperature;
Steps:
1 Preparation of 3,4-dimethoxythiophene from 3,4-dibromothiophene
21 g of sodium methoxide and 72 g of methanol were added to a 100 ml four-necked flask (the concentration of sodium methoxide relative to the methanol solvent was 22.6% by weight based on the total amount of sodium methoxide before the reaction) and dissolved at 70 under an argon atmosphere.After addition of 0.83 g of cuprous bromide, 15 g of 3,4-dibromothiophene was added dropwise, and the reaction solution became colorless transparent to black. After completion of the dropwise addition, 50 g of methanol was distilled off (sodium methoxide to methanol solvent The concentration was 48.8 wt% based on the total amount of sodium methoxide before the reaction)The reaction was heated to reflux at 97 .When the reaction was traced by gas chromatography, 3,4-dibromothiophene and 3-bromo-4-methoxythiophene were found to be below the detection limit at the reflux starting time of 5 hours.After water was added to the reaction mixture and the mixture was filtered, the crude product was extracted from toluene, and the toluene layer was washed with water, and then the toluene layer was dried with magnesium sulfate.After the magnesium sulfate was removed by filtration, the toluene layer was concentrated by a rotary evaporator and then subjected to vacuum distillation to obtain 7.28 g (yield: 81.5%) of 3,4-dimethoxythiophene. The purity of its 3,4-dimethoxythiophene was 98.01% by gas chromatography.The purity (concentration) by gas chromatography in the present invention was indicated by the area ratio of the peak area obtained by the detection device by FID using Agilent 6890N network GC manufactured by Aglient Technologies.
References:
Nippon Karitto Corporation;Koga, Minekaju;Nishiyama, Masaki;Yisikita, Yoshihito;Kiryu, Toshiyuki;Yamaguchi, Yoji KR101558628, 2015, B1 Location in patent:Paragraph 0184-0190

3141-26-2
311 suppliers
$5.00/1g

51792-34-8
321 suppliers
$6.00/1g

177364-96-4
0 suppliers
inquiry

51792-34-8
321 suppliers
$6.00/1g
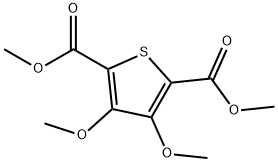
118851-98-2
0 suppliers
inquiry

51792-34-8
321 suppliers
$6.00/1g
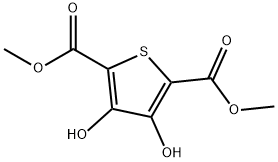
58416-04-9
97 suppliers
$52.00/250mg

77-78-1
319 suppliers
$19.69/1ml

51792-34-8
321 suppliers
$6.00/1g

118851-98-2
0 suppliers
inquiry