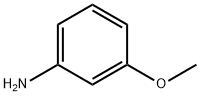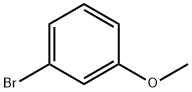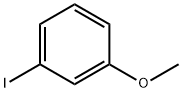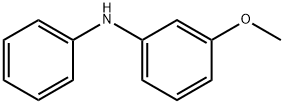
3-Methoxydiphenylamine synthesis
- Product Name:3-Methoxydiphenylamine
- CAS Number:101-16-6
- Molecular formula:C13H13NO
- Molecular Weight:199.25
Yield: 90%
Reaction Conditions:
with nickel(II) bromide trihydrate;N,N-dimethyl-cyclohexanamine;Eosin Y in 1,2-dimethoxyethane;N,N-dimethyl-formamide at 50; for 12 h;Inert atmosphere;
Steps:
15
General procedure: 3-Methylthioaniline (11.62 g, 83.5 mmol) and bromobenzene (8.74 g, 55.7 mmol) were added to a 250 mL reaction flask containing 100 mL of N,N-dimethylformamide under a nitrogen atmosphere. NiBr2·3H2O (0.30 g, 1.114 mmol) and photocatalyst 1a (10.50 mg, 0.011 mmol) were added in that order. Ethylene glycol dimethyl ether (0.10 g, 1.114 mmol), N,N-dimethylcyclohexylamine (12.76 g, 100.26 mmol), the reaction liquid was raised to 50 ° C, The reaction was carried out for 12 hours under blue light irradiation at a wavelength of 465 nm. After the reaction was completed, the light was stopped and heated, and the reaction flask was cooled to room temperature. The reaction solution is distilled under reduced pressure to remove N,N-dimethylformamide and N,N-dimethylcyclohexylamine; The residual liquid was diluted with n-hexane, and the insoluble inorganic salt in the residual liquid was removed by filtration, and the filtrate was concentrated. Distillation under reduced pressure gave 10.7 g of 3-methylthiodiphenylamine in a yield of 89%.
References:
Shaanxi Normal University;Huang Zhiyan;Xie Kun;Meng Ge;Ma Junjie;Xue Dong;Yang Jun CN108409618, 2018, A Location in patent:Paragraph 0035; 0060-0061