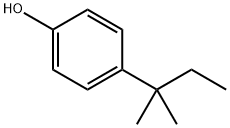
4-tert-Amylphenol synthesis
- Product Name:4-tert-Amylphenol
- CAS Number:80-46-6
- Molecular formula:C11H16O
- Molecular Weight:164.24
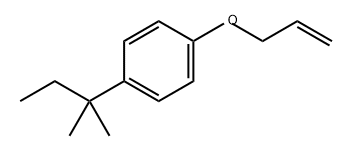
261618-80-8
1 suppliers
inquiry

80-46-6
300 suppliers
$6.00/10g
Yield:80-46-6 93%
Reaction Conditions:
Stage #1: 1-allyloxy-4-(1',1''-dimethylpropyl)benzenewith C12H37NiP4(1+)*C2F6NO4S2(1-) in tetrahydrofuran at 20; for 0.5 h;Glovebox;Schlenk technique;Inert atmosphere;
Stage #2: with toluene-4-sulfonic acid in tetrahydrofuran; for 15 h;Glovebox;Schlenk technique;Reflux;Inert atmosphere;
Steps:
Procedure C: Catalysis
General procedure: Inside a glovebox, a flame-dried 15 mL Schlenk tube was charged with [Ni(PMe3)4H](SO2CF3)2N (1.6 mg,2.48 μmol, 1 mol-%), and dry THF was added (0.16 M, 1.5 mL) using a Schlenk line. The O-allyl ether(0.25 mmol, 1 equiv) was then added under argon counter-flow. The reaction mixture was allowed to stirat room temperature for 30 minutes. This was followed by addition of pTsOH·H2O (1 equiv) and thereaction mixture was then heated to reflux for the time specified. The reaction was quenched by additionof ethyl acetate and water followed by transfer to a separating funnel. The aqueous phase was extractedwith ethyl acetate (3 × 5 mL), and the combined organic phases were then dried over MgSO4 and dried byrotary evaporation to give the crude alcohol, which was finally purified by column chromatography onsilica gel.
References:
Kathe, Prasad M.;Berkefeld, Andreas;Fleischer, Ivana [Synlett,2021,vol. 32,# 16,p. 1629 - 1632] Location in patent:supporting information
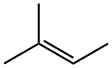
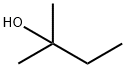
513-35-9
187 suppliers
$12.00/25g

108-95-2
785 suppliers
$14.00/25g

80-46-6
300 suppliers
$6.00/10g
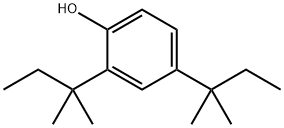
120-95-6
134 suppliers
$11.00/25g