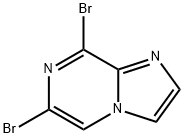
6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE synthesis
- Product Name:6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE
- CAS Number:63744-22-9
- Molecular formula:C6H3Br2N3
- Molecular Weight:276.92
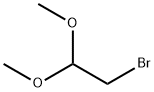
7252-83-7
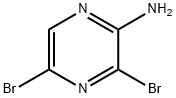
24241-18-7
![6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/63744-22-9.gif)
63744-22-9
1. 3,5-dibromopyrazin-2-amine (1018 g, 4.03 mol) was dissolved in water (10 kg) at room temperature. 2. 2-bromo-1,1-dimethoxyethane (1.79 kg, 4.14 mol) was added to the above solution and mixed with stirring. 3. The reaction mixture was heated to reflux and held for 2 hours. 4. After the reaction was complete, water (15.3 kg) and sodium bicarbonate (744 g) were added to the reaction solution and stirring was continued for 15 minutes. 5. The reaction mixture was filtered and the solid product was collected. 6. 6. After drying, 6,8-dibromoimidazo[1,2-A]pyrazine (1106 g, 3.99 mmol, 99% yield) was obtained as a brown solid. 7. The structure of the product was confirmed by 1H-NMR (400 MHz, DMSO-d6): δ 7.90 (s, 1H), 8.23 (s, 1H), 9.02 (s, 1H).

24241-18-7
442 suppliers
$6.00/5g
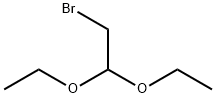
2032-35-1
492 suppliers
$5.00/10g
![6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/63744-22-9.gif)
63744-22-9
233 suppliers
$10.00/1g
Yield:63744-22-9 100%
Reaction Conditions:
in tetrahydrofuran;water at 20; for 19 h;Reflux;
Steps:
1-1
Intermediate Example 1 -1 : Preparation of 6,8-dibromo-imidazo[ 1 ,2-a]pyrazineTo a stirred suspension of 2-amino-3,5-dibrompyrazine (427 g, 1688mmol) in water (6.4 L) / THF (482 mL), at rt was added bromacetaldehyde-diethylacetal (998 g, 5065 mmol) in one portion. After stirring under reflux for 4 h, the clear orange solution was stirred for an additional 15 h at rt. The suspension was filtered, and the remaining solid was washed with MeOH (2 L) and dried in vaccuo at 60° C to yield 6,8-dibromo-imidazo[1 ,2-a]pyrazine as an off-white solid (500 g, 107% with residual MeOH): 1 H-NMR (300 MHz, d6-DMSO): δ =9.02 (s, 1 H), 8.23 (d, 1 H), 7.89 (d, 1 H) ppm. UPLC-MS: RT = 0.80 min; m/z 277.9 [MH+]; required MW = 276.9.
References:
BAYER PHARMA AKTIENGESELLSCHAFT;KOPPITZ, Marcus;KLAR, Ulrich;JAUTELAT, Rolf;KOSEMUND, Dirk;BOHLMANN, Rolf;BADER, Benjamin;LIENAU, Philip;SIEMEISTER, Gerhard WO2012/80236, 2012, A1 Location in patent:Page/Page column 63

7252-83-7
418 suppliers
$5.00/10g

24241-18-7
442 suppliers
$6.00/5g
![6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/63744-22-9.gif)
63744-22-9
233 suppliers
$10.00/1g
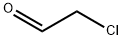
107-20-0
0 suppliers
$18.10/5ml

24241-18-7
442 suppliers
$6.00/5g
![6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/63744-22-9.gif)
63744-22-9
233 suppliers
$10.00/1g

24241-18-7
442 suppliers
$6.00/5g

17157-48-1
31 suppliers
inquiry
![6,8-DIBROMOIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/63744-22-9.gif)
63744-22-9
233 suppliers
$10.00/1g