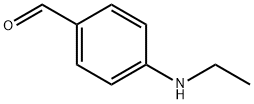
Benzaldehyde, 4-(ethylamino)- (9CI) synthesis
- Product Name:Benzaldehyde, 4-(ethylamino)- (9CI)
- CAS Number:79865-89-7
- Molecular formula:C9H11NO
- Molecular Weight:149.19
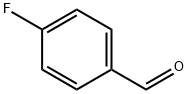
459-57-4
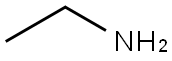
75-04-7

79865-89-7
Using 4-fluorobenzaldehyde (12.4 g, 0.1 mol) and 60% aqueous ethylamine (50 mL, 0.67 mol) as raw materials, the reaction was carried out in a 100 mL autoclave with a stir bar, sealed, and then placed in an oil bath at 130 °C with electromagnetic stirring for 15 h. The mixture was extracted with ethyl acetate three times (50 mL each) and washed with 10% hydrochloric acid twice (50 mL each). After completion of the reaction, the mixture was extracted with ethyl acetate three times (50 mL each time), the organic phases were combined and washed with 10% hydrochloric acid twice (50 mL each time). Subsequently, the pH of the aqueous phase was adjusted to 9 with 10% sodium carbonate solution, and the mixture was again extracted with ethyl acetate three times (30 mL each time), the organic phases were combined and dried with anhydrous sodium sulfate. After drying, the solvent was removed by rotary evaporator to obtain the crude product. The crude product was purified by silica gel column chromatography (200-300 mesh) with dichloromethane as eluent to give 4-(ethylamino)benzaldehyde as a light yellow solid (4 g, 30% yield). The product was analyzed by high resolution mass spectrometry (EI) and the calculated value of C9H11NO was 149.0841 and the measured value of [M + H]+ was 149.0840.

64-17-5
813 suppliers
$10.00/50g
555-16-8
560 suppliers
$5.00/5g

79865-89-7
51 suppliers
$53.00/100mg
Yield:79865-89-7 97.3%
Reaction Conditions:
with hydrogen in ethanol at 160; under 15001.5 Torr; for 7 h;
Steps:
2.5 Catalytic reactions and product analysis
The nitroarenes (0.81mmol), alcohols and the as-synthesized catalysts were placed in reactor (100mL). Before hydrogenation-alkylation tandem experiments, the reactor was purged with N2 and H2 to remove the air and N2 for three times, respectively. The reaction mixture were isolated by the magnet, and the isolated NiCo/MgO-C catalyst was washed with ethanol and dried before reused for recyclability measurement. The conversions and yields of reactions of hydrogenation-alkylation tandem reaction were analyzed by using GC-MS.
References:
Li, Weizuo;Wang, Jing;He, Dafang;He, Guangyu;Chen, Haiqun [Molecular catalysis,2022,vol. 529,art. no. 112520]

459-57-4
594 suppliers
$6.00/25g

75-04-7
405 suppliers
$10.00/20mg

79865-89-7
51 suppliers
$53.00/100mg
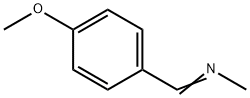
13114-23-3
11 suppliers
inquiry

75-04-7
405 suppliers
$10.00/20mg

79865-89-7
51 suppliers
$53.00/100mg
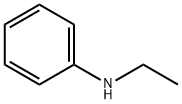
103-69-5
272 suppliers
$13.00/100g

79865-89-7
51 suppliers
$53.00/100mg
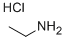
557-66-4
274 suppliers
$26.00/25g
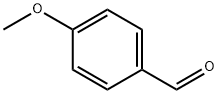
123-11-5
875 suppliers
$10.00/10g

79865-89-7
51 suppliers
$53.00/100mg