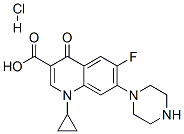
Ciprofloxacin hydrochloride synthesis
- Product Name:Ciprofloxacin hydrochloride
- CAS Number:93107-08-5
- Molecular formula:C17H18FN3O3.HCl
- Molecular Weight:367.8
a) cyclopropyl carboxylic acid is added in the organic solvent, and the adding piperazine, reacted 3~8 hours down as acid binding agent with alkali at 60~120 ℃, the monitoring cyclopropyl carboxylic acid is residual less than 2% o'clock termination reaction, stirring is cooled to 0~30 ℃, leaves standstill suction filtration;
b) add water and hydrochloric acid in the products therefrom toward a) step, under agitation be warming up to molten clearly, add activated carbon decolorizing, filtered while hot, and transfer filtrate pH to 2~4 with hydrochloric acid, and the cooling crystallization filters, rinsing, and drying makes ciprofloxacin HCl.
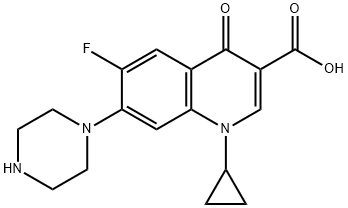
85721-33-1
615 suppliers
$5.00/1g

93107-08-5
311 suppliers
$28.00/5g
Yield:93107-08-5 95%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;dichloromethane at 20; for 1 h;
Steps:
Synthesis of 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid hydrochloride (1.6)
Compound 2.1 (iso mg, 0.45 mmol, 1 eq) was stirred in DCM ( mL) for minutes.Then 4M HC1 in dioxane (2.26 mL, 9.05 mmol, 20 eq) was added dropwise and themixture stirred for i hour. Upon completion, the mixture was washed with hexane (3 xi mL) and lyophilized overnight to give compound 1.6 (>95%) as a pale brown solid. 1H NMR (400 MHz, DMSO-d6) 6 15.20 (br. s., 1H), 9.46 (br. s., 2H), 8.67 (s, 1H), 7.94 (d, J = 13.09 Hz, 1H), 7.61 (d, J = 7.30 Hz, 1H), 3.86 (br. s., 1H), 3.57 (br. 5., 4H), 3.31 (br. 5., 4H), 1.29 - 1.35 (m, 2H), 1.15 - 1.24 (m, 3H); HR1VJS Observed 332.1404 [M+H] theoretical value 332.1405 [M+H] JR (Umax/cm1) 1701, 1624, 1491, 1458, 1383, 1341, 1272, 1142, iio6, 1034, 941, 909, 889, 853, 829, 804, 774, 749, 703, 66, 636, 619; LC25 MS Retention time 4.70 minutes, found 332.0 [M+H] calculated for C17H18FN303332.35 [M+H].
References:
WO2018/220365,2018,A1 Location in patent:Page/Page column 97
86393-32-0
434 suppliers
$5.00/100mg

93107-08-5
311 suppliers
$28.00/5g

110-85-0
588 suppliers
$5.00/5G
93107-30-3
165 suppliers
$11.00/250mg

93107-08-5
311 suppliers
$28.00/5g

110-85-0
588 suppliers
$5.00/5G
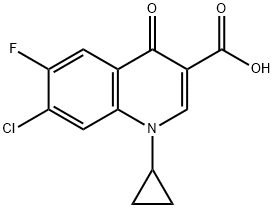
86393-33-1
346 suppliers
$15.00/25g
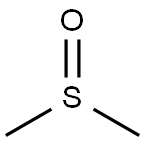
67-68-5
1327 suppliers
$15.00/100g

85721-33-1
615 suppliers
$5.00/1g

93107-08-5
311 suppliers
$28.00/5g
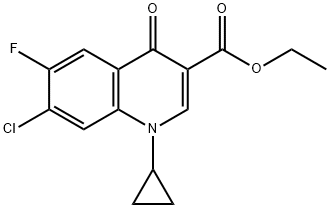
86483-54-7
11 suppliers
inquiry

93107-08-5
311 suppliers
$28.00/5g