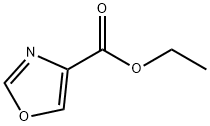
Ethyl oxazole-4-carboxylate synthesis
- Product Name:Ethyl oxazole-4-carboxylate
- CAS Number:23012-14-8
- Molecular formula:C6H7NO3
- Molecular Weight:141.12
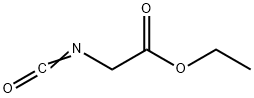
2949-22-6

23012-14-8
The general procedure for the synthesis of ethyl oxazole-4-carboxylate from ethyl isocyanoylacetate was as follows: formic acid (8.62 mL, 228 mmol) was added in batches to anhydrous THF (200 mL), and the phenomenon of gas escape was observed. The mixture was stirred for 30 min to form a sol. Subsequently, a solution of ethyl isocyanoacetate (25 mL, 228 mmol) and triethylamine (Et3N, 60.5 mL, 434 mmol) was added to the reaction mixture. The mixture was stirred at room temperature for 1 hour and then changed to reflux conditions overnight. After the reaction was completed, the mixture was cooled to room temperature. Water was added and the mixture was extracted with ether (Et2O, 3 times). The organic phases were combined, dried with magnesium sulfate (MgSO4), filtered and concentrated. The residue was purified by fast column chromatography (FC, eluent: ethyl acetate/heptane, ratio graded from 1:5 to 1:3 and finally to 1:1) to afford ethyl oxazole-4-carboxylate (27.2 g, 84% yield). LC-MS analysis resulted in a retention time (tR) = 0.58 min and a mass-to-charge ratio in the electrosprayed positive ion mode (ES+) (m/z) = 142.07 .

2949-22-6
154 suppliers
$20.00/1g

23012-14-8
209 suppliers
$5.00/250mg
Yield:23012-14-8 84%
Reaction Conditions:
Stage #1: 1,1'-carbonyldiimidazolewith formic acid in tetrahydrofuran; for 0.5 h;
Stage #2: Glycine ethyl ester isocyanatewith triethylamine in tetrahydrofuran at 20; for 1 h;Heating / reflux;
Steps:
To a sol. of formic acid (8.62 mL, 228 mmol) in dry THF (200 mL) was added portionwise CDI (37.05 g, 228 mmol), whereas a gas evolution occurred. The mixture was stirred for 30 min, and a sol. of ethyl isocyanoacetate (25 mL, 228 mmol) in Et3N (60.5 mL, 434mmol) was added to the reaction mixture. The mixture was stirred at rt for 1 h, then under reflux overnight. The reaction mixture was allowed to cool to rt. Water was added, and the mixture was extracted with Et2O (3x). The combined org. extracts were dried over MgSO4, filtered and concentrated. Purification of the residue by FC (EtOAc / heptane; 1:5 -> 1:3 - 1:1) yielded the title compound (27.2 g, 84%). LC-MS: tR = 0.58, ES+: 142.07.
References:
WO2006/21402,2006,A1 Location in patent:Page/Page column 33-34
177760-52-0
214 suppliers
$6.00/250mg

23012-14-8
209 suppliers
$5.00/250mg

64-18-6
1131 suppliers
$22.12/250ML
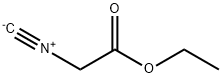
2999-46-4
217 suppliers
$13.43/1gm:

23012-14-8
209 suppliers
$5.00/250mg
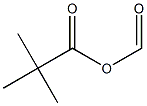
10535-67-8
1 suppliers
inquiry

2999-46-4
217 suppliers
$13.43/1gm:

23012-14-8
209 suppliers
$5.00/250mg
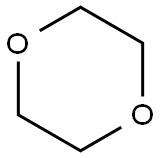
123-91-1
709 suppliers
$17.00/25g
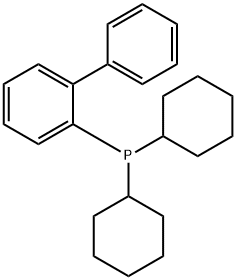
247940-06-3
276 suppliers
$6.00/1g

23012-14-8
209 suppliers
$5.00/250mg