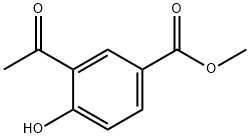
METHYL 3-ACETYL-4-HYDROXYBENZOATE synthesis
- Product Name:METHYL 3-ACETYL-4-HYDROXYBENZOATE
- CAS Number:57009-12-8
- Molecular formula:C10H10O4
- Molecular Weight:194.18

111-34-2
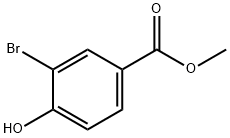
29415-97-2

57009-12-8
Step 2: To a degassed ethanol (3 L) solution of methyl 3-bromo-4-hydroxybenzoate (350 g, 1514.87 mmol) under nitrogen protection were sequentially added triethylamine (0.528 L, 3787.17 mmol), 1-(vinyloxy)butane (0.588 L, 4544.60 mmol), 1,1 '-bis(diphenylphosphino) ferrocene (33.1 g, 60.6 mmol) and diacetoxypalladium (8.50 g, 37.9 mmol). The reaction mixture was stirred at 70 °C overnight. After completion of the reaction, the mixture was cooled to room temperature, filtered and the filtrate was concentrated. The resulting solid was dissolved with dichloromethane (2 L), followed by the slow addition of 4 N hydrochloric acid (1.14 L, 4544 mmol) under stirring. After continued stirring for 2 h, the organic phase was separated, dried with anhydrous magnesium sulfate, filtered and concentrated to give the crude product. The crude product was dissolved in ether (5 L), stirred for 2 h, filtered, and the filtrate was concentrated to dryness to give methyl 3-acetyl-4-hydroxybenzoate (240 g, 82% yield) as a beige powder. Mass spectral data: [MH]- 193.

111-34-2
267 suppliers
$13.00/25mL

29415-97-2
172 suppliers
$5.00/1g

57009-12-8
57 suppliers
$30.00/100mg
Yield: 82%
Reaction Conditions:
Stage #1:-butyl vinyl ether;methyl 3-bromo-4-hydroxybenzoate with triethylamine;(diphenylphosphin)ferrocene;palladium diacetate in diethyl ether;ethanol at 70;Inert atmosphere;
Stage #2: with hydrogenchloride;water in dichloromethane for 2 h;
Steps:
1.00.2
Step 2 To a degassed solution of methyl 3-bromo-4-hydroxybenzoate (350 g, 1514.87 mmol) in ethanol (3 L) were added triethylamine (0.528 L, 3787.17 mmol), 1-(vinyloxy)butane (0.588 L, 4544.60 mmol), 1,1'-bis(diphenylphosphino)ferrocene (33.1 g, 60.6 mmol) and diacetoxypalladium (8.50 g, 37.9 mmol) under nitrogen. The mixture was heated at 70° C. overnight. The reaction was cooled down, filtered and the filtrate concentrated. The resulting solid was solubilized with DCM (2 L) and HCl 4N (1.14 L, 4544 mmol) was added under stirring. Stirring was maintained for 2 hrs, the organic phase was separated, dried over magnesium sulfate, filtered and concentrated to afford a solid which was stirred in diethyl ether (5 L) for 2 hrs. The solid was filtered off and the filtrate concentrated to dryness to afford methyl 3-acetyl-4-hydroxybenzoate (240 g, 82%) as a beige powder. Mass spectrum: [M-H]- 193.
References:
ASTRAZENECA AB US2011/98271, 2011, A1 Location in patent:Page/Page column 39

67-56-1
790 suppliers
$7.29/5ml-f
16357-40-7
65 suppliers
$19.00/100mg

57009-12-8
57 suppliers
$30.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
1450-75-5
305 suppliers
$10.00/1g

57009-12-8
57 suppliers
$30.00/100mg
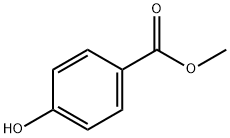
99-76-3
815 suppliers
$5.00/25g

57009-12-8
57 suppliers
$30.00/100mg
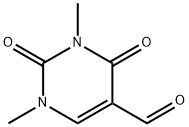
4869-46-9
87 suppliers
$27.00/100mg
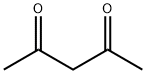
123-54-6
588 suppliers
$10.00/25ml

57009-12-8
57 suppliers
$30.00/100mg