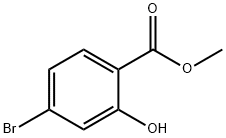
METHYL 4-BROMO-2-HYDROXYBENZOATE synthesis
- Product Name:METHYL 4-BROMO-2-HYDROXYBENZOATE
- CAS Number:22717-56-2
- Molecular formula:C8H7BrO3
- Molecular Weight:231.04

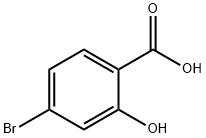
67-56-1
1666-28-0

22717-56-2
2-Hydroxy-4-bromobenzoic acid (5.0 g, 23.1 mmol) was dissolved in 20 mL of anhydrous methanol and 1.7 mL of concentrated sulfuric acid was added slowly. The reaction mixture was heated and refluxed under stirring for 24 hours. Upon completion of the reaction, the reaction solution was poured into ice water and concentrated by rotary evaporator to remove methanol. Subsequently, extraction was carried out with ethyl acetate (EA) and the organic phase was washed with saturated sodium bicarbonate (NaHCO3) solution and dried over anhydrous sodium sulfate. Finally, the organic phase was concentrated to afford methyl 2-hydroxy-4-bromobenzoate (5.31 g) as a reddish brown solid, and the product was used directly in the next reaction without further purification.

1666-28-0
216 suppliers
$9.00/1g

22717-56-2
129 suppliers
$6.00/250mg
Yield: 93%
Reaction Conditions:
with sulfuric acid in methanol for 19 h;Reflux;
Steps:
2 Methyl 4-bromo-2-hydroxybenzoate (INT1)
A solution of 2-hydroxy-4-bromobenzoic acid (4.57 g, 1.0 equiv., 20.0 mmol) in anhydrous MeOH (60 ml_) was transferred to a flame-dried one-necked flask (100 ml_) equipped with a stirrer bar. Subsequently, addition of concentrated sulfuric acid (98.5%, 2.2 ml_) was performed. The mixture was stirred at reflux for 19 hours before being concentrated. After evaporation of the solvent, addition of EtOAc (60 ml_) and careful addition of saturated aqueous NaHCC (80 ml_) was made, and the phases were separated. The aqueous phase was extracted with EtOAc (3x50 ml_) and the organic phase was washed with brine (100 ml_), before being dried over sodium sulphate, filtered and concentrated to yield a dark brown crude isolate. The crude material was purified by flash column chromatography on silica gel using an eluent system of Heptane/EtOAc 90: 10 to afford the desired product as colorless needles (4.31g, 18.7 mmol, 93%). TLC (Heptane: EtOAc, 90: 10 v/v, UV, KMn04): Rf= 0.38; .H NMR (400 MHz, CDCb): d 10.83 (s, 1H), 7.68 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 1.9 Hz, 1H), 7.02 (dd, J = 5.5 Hz, 1.9 Hz, 1H), 3.95 (s, 3H);13C NMR (101 MHz, CDCb): d 170.3, 162.1, 131.0, 130.1, 122.9, 121.0, 111.5, 52.7; IR (ATR, CH2CI2): 3155, 2954, 1677, 1606, 1570, 1480, 1439, 1330, 1282, 1204,1095, 885, 772, 1765 cm 1; HRMS (m/z): [M] calcd. for CsHeBrC, 228.9506; found, 228.9516.
References:
AARHUS UNIVERSITET WO2021/110968, 2021, A1 Location in patent:Page/Page column 39; 40

67-56-1
790 suppliers
$7.29/5ml-f

1666-28-0
216 suppliers
$9.00/1g

22717-56-2
129 suppliers
$6.00/250mg
139102-34-4
147 suppliers
$5.00/250mg

22717-56-2
129 suppliers
$6.00/250mg

1666-28-0
216 suppliers
$9.00/1g

18107-18-1
210 suppliers
$33.00/5g

22717-56-2
129 suppliers
$6.00/250mg
65-49-6
510 suppliers
$6.00/25g

22717-56-2
129 suppliers
$6.00/250mg