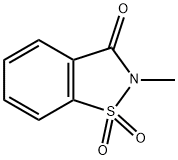
N-METHYLSACCHARIN synthesis
- Product Name:N-METHYLSACCHARIN
- CAS Number:15448-99-4
- Molecular formula:C8H7NO3S
- Molecular Weight:197.21

67-56-1
790 suppliers
$7.29/5ml-f
81-07-2
465 suppliers
$13.98/25G

15448-99-4
90 suppliers
$26.00/250mg
Yield: 92%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 0 - 20;regioselective reaction;Mitsunobu Displacement;
Steps:
Alkylation of saccharin under Mitsunobu conditions, general procedure
General procedure: Diisopropyl azodicarboxylate (DIAD, 4.5 mmol) was placed in a 50 mL round-bottomed flask equipped with a stirring bar. Then triphenylphosphine (Ph3P, 4.5 mmol) and dry tetrahydrofuran (THF, 20 mL) were added and the solution was cooled in an ice bath. Then the alcohol(3.0 mmol) was added. The mixture was then stirred for 10 minand a solution of saccharin (3.0 mmol) in dry THF (5 mL) was added dropwise. After being stirred for 30 min, the flask was removed from the ice bath and the solution was stirred at room temperature, and monitored by TLC. When the reaction was judged to be complete, the organic solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel using petroleum ether/EtOAc (v/v = 10:1) as the eluent to give the products. All the isolated products were characterised by physical and spectroscopic data and also by comparison with authentic samples. 5,7-10 The physicaland spectral data of the products are shown as follows. 2-Methyl-1,2-benzisothiazol-3(2H)-one 1,1-dioxide: Colourless solid,m.p. 132-133 °C (lit.7 129-130 °C); 1H NMR (400 MHz, CDCl3):δ 3.26 (s, 3H), 7.83 (m, 2H), 7.93 (dd, J = 7.2, 1.6 Hz, 1H), 8.05 (dd,J = 7.2, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 23.2, 120.9,125.1, 127.5, 134.3, 134.6, 137.5, 158.6.
References:
Wang, Xiaolong;Ma, Yanying;Ju, Tingting [Journal of Chemical Research,2013,vol. 37,# 7,p. 417 - 419]
2527-66-4
110 suppliers
inquiry

15448-99-4
90 suppliers
$26.00/250mg
128-44-9
451 suppliers
$24.00/25g

74-88-4
357 suppliers
$15.00/10g

15448-99-4
90 suppliers
$26.00/250mg

128-44-9
451 suppliers
$24.00/25g

77-78-1
319 suppliers
$19.69/1ml

15448-99-4
90 suppliers
$26.00/250mg
75-91-2
217 suppliers
$26.00/100g

81-07-2
465 suppliers
$13.98/25G

15448-99-4
90 suppliers
$26.00/250mg