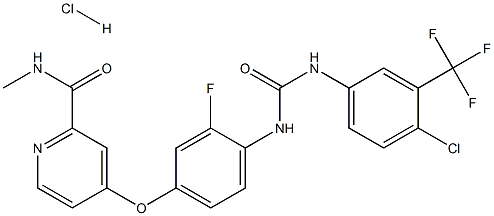
Regorafenib (Hydrochloride) synthesis
- Product Name:Regorafenib (Hydrochloride)
- CAS Number:835621-07-3
- Molecular formula:C21H16Cl2F4N4O3
- Molecular Weight:519.2764
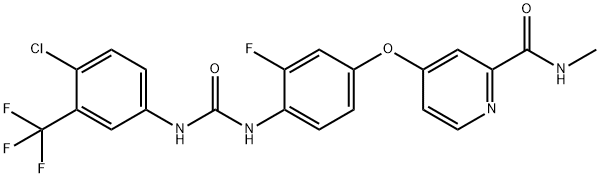
755037-03-7

835621-07-3
GENERAL STEPS: 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpyridine amide (free base, 2.0 g) was dissolved in anhydrous tetrahydrofuran (15 mL) and 4M HCl/dioxane solution was slowly added (to excess). Upon completion of the reaction, the mixed solution was concentrated under reduced pressure to give 2.32 g of off-white solid. The resulting crude product was dissolved in preheated ethanol (125 mL), appropriate amount of activated carbon was added and heated to reflux for 15 minutes. Subsequently, the hot suspension was filtered through a diatomaceous earth pad (model 521) and cooled to room temperature. The filtrate was placed in a refrigerator overnight to promote crystallization. On the following day, the crystallized product was collected by diafiltration, washed sequentially with cold ethanol and hexane, and air-dried. The mother liquor was further concentrated and the above crystallization process was repeated (overnight in a refrigerator) to collect the second batch of crystalline product and combined with the first batch. Finally, the resulting colorless hydrochloride product was dried in a vacuum oven at 60°C for two days to give a final product of 1.72 g (79% yield). Melting point: 215°C. Elemental analysis (calculated/measured): C 48.57/48.68, H 3.11/2.76, N 10.79/10.60, Cl 13.65/13.63, F 14.63/14.88.

755037-03-7
482 suppliers
$25.00/5mg

835621-07-3
124 suppliers
inquiry
Yield:835621-07-3 79%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;1,3-dioxane;
Steps:
2
The compound of example 1 as a free base (2.0 g) was dissolved in anhydrous tetrahydrofuran (15 mL) and a 4M HCl/dioxane was added (excess). The solution was then concentrated in vacuo to afford 2. 32 grams of off-white solids. The crude salt was dissolved in hot ethanol (125 mL), activated carbon was added and the mixture heated at reflux for 15 minutes. The hot suspension was filtered through a pad of Celite 521 and allowed to cool to room temperature. The flask was placed in a freezer overnight. The crystalline solids were collected by suction filtration, washed with ethanol, then hexane and air-dried. The mother liquors were concentrated down and crystallization (in freezer) allowed taking place overnight. A second crop of solids was collected and combined with the first crop. The colorless salt was dried in a vacuum oven at 60 °C over two days. Yield of hydrochloride salt obtained 1.72 g (79%). Melting point: 215 °C Elemental analysis: CALCD. Found C 48.57 48.68 H 3.11 2.76 N 10.79 10.60 Cl 13.65 13.63 F 14.63 14.88
References:
WO2005/9961,2005,A2 Location in patent:Page/Page column 47-48
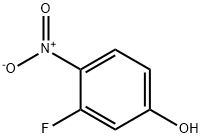
394-41-2
299 suppliers
$16.00/1g

835621-07-3
124 suppliers
inquiry
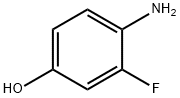
399-95-1
433 suppliers
$14.00/5g

835621-07-3
124 suppliers
inquiry
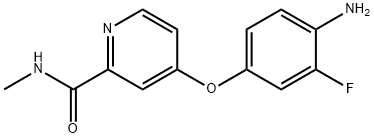
757251-39-1
247 suppliers
$11.00/250mg

835621-07-3
124 suppliers
inquiry