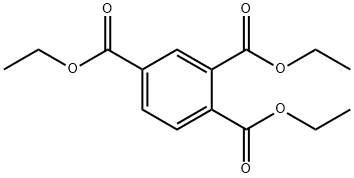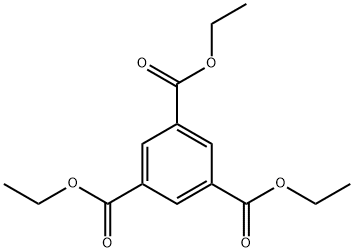
Triethyl 1,3,5-benzenetricarboxylate synthesis
- Product Name:Triethyl 1,3,5-benzenetricarboxylate
- CAS Number:4105-92-4
- Molecular formula:C15H18O6
- Molecular Weight:294.3
Yield: 92%
Reaction Conditions:
with bis(1,5-cyclooctadiene)nickel(0);triphenylphosphine in toluene at 23; for 2 h;Inert atmosphere;
Steps:
2 Example 2 Catalytic [2+2+2] Cyclotrimerization of Ethyl Propiolate
Under an argon atmosphere, Ni(COD)2 (5.5 mg, 0.020 mmol; Acros, Pittsburgh, Pa.; catalog No. 223970050) and PPh3 (15.6 mg, 0.060 mmol; Sigma-Aldrich Co., St. Louis, Mo.; catalog No. T84409) were mixed in 10 mL of toluene at 23° C.
The resulting mixture was stirred at this temperature for 5 min to produce a stock solution of the catalyst.
A portion of the stock solution (375 μL) was added to a 10 mL scintillation vial under an argon atmosphere and the volume was brought to 2 mL with toluene, followed by the addition of ethyl propiolate (152 μL, 1.5 mmol).
The reaction was complete within 2 h, producing a mixture of 1,2,4- and 1,3,5-isomers in a 97:3 molar ratio.
The 1,2,4-isomer (triethyl trimellitate) was separated from the isomeric mixture using column chromatography (eluted with diethyl ether/hexanes) and isolated in 92% yield. 1H NMR (400 MHz, CDCl3, δ): 8.41 (1H, d, 4JH-H=1.7 Hz, ArH), 8.20 (1H, dd, 3JH-H=7.9 Hz, 4JH-H=1.7 Hz, ArH), 7.76 (1H, d, 3JH-H=7.9 Hz, ArH), 4.50-4.35 (6H, m, CH2), 1.50-1.35 (9H, m, CH3); 13C{1H} NMR (101 MHz, CDCl3, δ): 167.24, 166.70, 165.07, 136.36, 132.77, 132.17, 132.10, 130.19, 128.95, 62.09, 62.03, 61.78, 14.36, 14.20, 14.15.
References:
The Procter & Gamble Company;Hayes, Jeffrey Charles;Guan, Hairong;Collias, Dimitris Ioannis US2016/264506, 2016, A1 Location in patent:Paragraph 0036
140-88-5
559 suppliers
$12.00/100ml

4105-92-4
85 suppliers
$32.20/5g
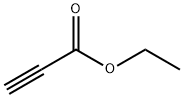
623-47-2
352 suppliers
$10.00/1g

4105-92-4
85 suppliers
$32.20/5g
33142-25-5
12 suppliers
inquiry

4105-92-4
85 suppliers
$32.20/5g