| Identification | More | [Name]
Miconazole | [CAS]
22916-47-8 | [Synonyms]
1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1h-imidazole
1-[2,4-DICHLORO-BETA-([2,4-DICHLOROBENZYL]OXY)-PHENETHYL]IMIDAZOLE
LABOTEST-BB LT00255845
MICONAZOLE
MICONAZOLE BASE
MONISTAT IV
1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-imidazol
1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-imidazol
1-[2-[(2,4-Dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole
Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-
Imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-
micronazol
Minostate
mjr1762
R 18134
r18134
MICONAZOLE USP BASE
MICONAZOLE FREE BASE
MICONAZOLE MM(CRM STANDARD)
MICONAZOLE USP(CRM STANDARD) | [EINECS(EC#)]
245-324-5 | [Molecular Formula]
C18H14Cl4N2O | [MDL Number]
MFCD00216019 | [Molecular Weight]
416.13 | [MOL File]
22916-47-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White or almost white powder. | [Melting point ]
159-163C | [Boiling point ]
555.1±50.0 °C(Predicted) | [density ]
1.5326 (rough estimate) | [refractive index ]
1.6200 (estimate) | [RTECS ]
NI4770000 | [Fp ]
87°(189°F) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Very slightly soluble in water, freely soluble in methanol, soluble in ethanol (96 per cent). | [form ]
neat | [pka]
pKa 6.91(H2O,t =25) (Uncertain) | [color ]
White to Off-White | [Water Solubility ]
Freely soluble in alcohols or acetone. Also soluble in DMF or chloroform. Insoluble in water | [Sensitive ]
Light Sensitive | [Major Application]
pharmaceutical (small molecule) | [InChI]
1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | [InChIKey]
BYBLEWFAAKGYCD-UHFFFAOYSA-N | [SMILES]
Clc1c(ccc(c1)Cl)C(OCc3c(cc(cc3)Cl)Cl)C[n]2cncc2 | [CAS DataBase Reference]
22916-47-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Miconazole(22916-47-8) | [EPA Substance Registry System]
1H-Imidazole, 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]- (22916-47-8) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing . | [RIDADR ]
3249 | [WGK Germany ]
3 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2933290000 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Chronic 4 |
| Hazard Information | Back Directory | [Chemical Properties]
White or almost white powder. | [Originator]
Daktarin,Janssen,Italy,1974 | [Uses]
Antifungal;Sterol 14-demethylase inhibitor.
Miconazole (Monistat-Derm, Micatin, etc.) is a synthetic imidazole antifungal compound that acts by altering cell membrane permeability. It is effective against most dermatophyte species, P. orbiculare, and C. albicans.

| [Uses]
Miconazole is an antifungal agent of the imidazole type. It is used in topical and vaginal preparations to prevent growth of dermatophytes, yeast, and molds. | [Uses]
Miconazole, is used as an antifungal inhibitor of aromatase. Miconazole has been shown to promote remyelination of neurons in chronic progressive multiple sclerosis mouse models. Miconazole is mainly used externally for the treatment of athlete's foot, ringworm, and jock itch. Internal application is used for oral or vaginal thrush (yeast infection). The oral gel may also be used for the lip disorder angular cheilitis. It is also used in photography. | [Definition]
ChEBI: 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole is a member of the class of imidazoles that is 1-(2,4-dichlorophenyl)-2-(imidazol-1-yl)ethanol in which the hydroxyl hydrogen is replaced by a 2,4-dichlorobenzyl group. It is an ether, a member of imidazoles and a dichlorobenzene. | [Indications]
Miconazole (Monistat) is a broad-spectrum imidazole
antifungal agent used in the topical treatment of cutaneous
dermatophyte infections and mucous membrane
Candida infections, such as vaginitis. Minimal absorption
occurs from skin or mucous membrane surfaces.
Local irritation to skin and mucous membranes can occur
with topical use; headaches, urticaria, and abdominal
cramping have been reported with treatment for
vaginitis. | [Manufacturing Process]
Imidazole is reacted with ω-bromo-2,4-dichloroacetophenone and that product
reduced with sodium borohydride.
A suspension of 10.3 parts of the α-(2,4-dichlorophenyl)imidazole-1-ethanol
thus obtained and 2.1 parts of sodium hydride in 50 parts of dry
tetrahydrofuran is stirred and refluxed for 2 hours. After this reaction time,
the evolution of hydrogen is ceased. Then there are added successively 60
parts dimethylformamide and 8 parts of 2,4-dichlorobenzyl chloride and
stirring and refluxing are continued for another 2 hours. The tetrahydrofuran
is removed at atmospheric pressure. The dimethylformamide solution is
poured onto water.
The product, 1-[2,4-dichloro-β-(2,4 -dichlorobenzyloxy)phenethyl]imidazole, is
extracted with benzene. The extract is washed with water, dried, filtered and
evaporated in vacuo. From the residual oily free base, the nitrate salt is
prepared in the usual manner in 2-propanol by treatment with concentrated
nitric acid, yielding, after recrystallization of the crude solid salt from a
mixture of 2-propanol, methanol and diisopropyl ether, 1-[2,4-dichloro-β-
dichlorobenzyloxy)phenethyl]imidazole nitrate; melting point 170.5°C. | [Brand name]
M-Zole (Actavis); Monistat (Ortho-McNeil); Monistat (Personal Products); Monistat (Johnson & Johnson). | [Therapeutic Function]
Antifungal | [Clinical Use]
Antifungal agent | [Synthesis]
Miconazole, 1-[2,4-dichloro-|?-[(2,4-dichlorobenzyl)oxy]phenethyl]-imidazole
(35.2.7), like ketoconazol, is synthesized from 2,4-dichlorophenacylbromide, which is reacted with imidazole to make 1-(2,4-dichlorobenzoylmethyl)-imidazole[2,4-dichloro-|?-(1-imida�zolyl)-acetophenone] (35.2.5). Reducing the carbonyl group in this molecule with sodium
borohydride gives 1-(2,4-dichlorophenyl)-3-(1-imidazolyl)-ethanol (35.2.6), and the hydroxyl
group is alkylated by 2,4-dichlorobenzylbromide using a powerful base such as sodium
hydride to make miconazole (35.2.7).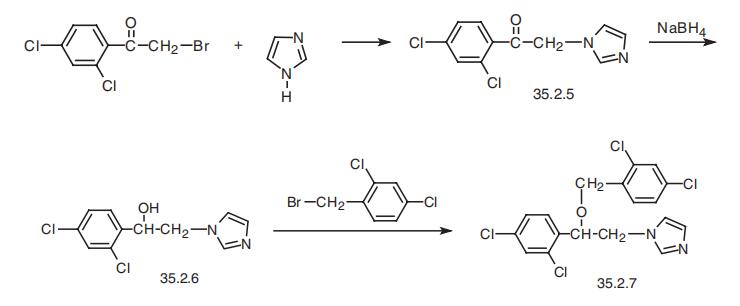
| [Veterinary Drugs and Treatments]
Miconazole is a broad spectrum imidazole antifungal agent with
some antibacterial activity. Miconazole will penetrate the intact
corneal epithelium. Topical miconazole therapy has been a favorite
first choice agent for treatment of fungal keratitis in the horse
by veterinary ophthalmologists for several years. Miconazole may
be delivered by subconjunctival route, but with some local irritation,
and topical use is the most commonly employed treatment
method. | [Veterinary Drugs and Treatments]
Topical miconazole has activity against dermatophytes and yeasts; miconazole shampoos can be effective treatment for Malassezia dermatitis.
Patients with severe, generalized infections may require systemic therapy. Lotions, sprays and creams are generally used for localized
lesions associated with Malassezia or dermatophytes. See otic section for information on application for Malassezia otitis externa.
Topical miconazole products are generally ineffective (or minimally effective) when used alone for dermatophytosis; adjunctive systemic
treatment is usually required.
Miconazole’s actions are a result of altering permeability of fungal cellular membranes and interfering with peroxisomal and mitochondrial
enzymes, leading to intracellular necrosis. Miconazole products are fungicidal with repeated application. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effect of coumarins enhanced. Antidepressants: avoid concomitant use with
reboxetine.
Antidiabetics: enhances hypoglycaemic effect
of gliclazide and glipizide; concentration of
sulphonylureas increased.
Antiepileptics: effect of fosphenytoin and phenytoin
enhanced; possibly increased carbamazepine
concentration.
Antihistamines: avoid with mizolastine, risk of
ventricular arrhythmias.
Antimalarials: avoid with piperaquine with artenimol
and artemether with lumefantrine
.
Antipsychotics: increased risk of ventricular
arrhythmias with pimozide - avoid; possibly
increased concentration of quetiapine - avoid.
Antivirals: concentration of saquinavir possibly
increased.
Ciclosporin: possibly increased ciclosporin
concentration.
Ergot alkaloids: increased risk of ergotism with
ergotamine and methysergide - avoid.
Sirolimus: concentration increased by miconazole.
Statins: possibly increased risk of myopathy
with atorvastatin and simvastatin - avoid with
simvastatin.
Tacrolimus: possibly increased tacrolimus
concentration | [Metabolism]
Miconazole is metabolised in the liver to inactive
metabolites; 10-20% of an oral dose is excreted in the
urine as metabolites. About 50% of an oral dose may be
excreted mainly unchanged in the faeces |
|
|