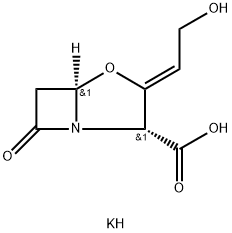
Potassium clavulanate
- CAS No.
- 61177-45-5
- Chemical Name:
- Potassium clavulanate
- Synonyms
- CLAVULANATE POTASSIUM;Clavulanate;Potassium Clavulanat;potassium calvulanate;Potassium clavulanate USP/EP/BP;MM 1415;Amonate;brl14151k;Clavulanate K;CLAVULANTE POTASSIUM
- CBNumber:
- CB2722502
- Molecular Formula:
- C8H10KNO5
- Molecular Weight:
- 239.27
- MDL Number:
- MFCD01710901
- MOL File:
- 61177-45-5.mol
| Melting point | >1600C (dec) |
|---|---|
| alpha | +55~+60° |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Freely soluble in water, slightly soluble in ethanol (96 per cent), very slightly soluble in acetone. |
| InChIKey | ABVRVIZBZKUTMK-JSYANWSFSA-M |
| CAS DataBase Reference | 61177-45-5(CAS DataBase Reference) |
| FDA UNII | Q42OMW3AT8 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H228-H252-H317-H334 | |||||||||
| Precautionary statements | P210-P235-P280-P302+P352-P304+P340+P312-P403+P235 | |||||||||
| Hazard Codes | F,Xn,Xi | |||||||||
| Risk Statements | 11-42/43-44-36-14 | |||||||||
| Safety Statements | 8-16-22-36/37-45-43-26 | |||||||||
| RIDADR | UN1325 - class 4.1 - PG 2 - Flammable solids, organic, n.o.s., HI: all | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | RN6802700 | |||||||||
| HS Code | 2941900000 | |||||||||
| NFPA 704 |
|
Potassium clavulanate price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 33454 | Potassium clavulanate VETRANAL | 61177-45-5 | 100mg | $199 | 2024-03-01 | Buy |
| Cayman Chemical | 19456 | Clavulanate (potassium salt) ≥95% | 61177-45-5 | 5mg | $29 | 2024-03-01 | Buy |
| Cayman Chemical | 19456 | Clavulanate (potassium salt) ≥95% | 61177-45-5 | 10mg | $51 | 2024-03-01 | Buy |
| Cayman Chemical | 19456 | Clavulanate (potassium salt) ≥95% | 61177-45-5 | 25mg | $78 | 2024-03-01 | Buy |
| Cayman Chemical | 19456 | Clavulanate (potassium salt) ≥95% | 61177-45-5 | 50mg | $99 | 2024-03-01 | Buy |
Potassium clavulanate Chemical Properties,Uses,Production
Description
Potassium clavulanate is a semi-synthetic beta-lactamase inhibitor isolated from Streptomyces. The compound contains a beta-lactam ring and strongly binds to beta-lactamase at their new active site, which protects other beta-lactam antibiotics such as amoxicillin from beta-lactamase catalysis.
Uses
Combination of potassium clavulanate and amoxicillin is normally used in medicine to treat numerous different infections caused by bacteria, for instance, pneumonia, sinusitis, ear infections, urinary tract infections, bronchitis, and skin infections. Potassium clavulanate can also combined with other penicillin-based antibiotics.
Cautions
Before taking a combination of potassium clavulanate with any other penicillin-based antibiotic, one should discuss with medical providers and let them know whether they have severe kidney disease, jaundice or liver problems. In addition, it is important to inform the physician if an individual is allergic to penicillin or cephalosporin antibiotic, such as Amoxil, Levaquin, omnicef, and cefzil.
Although it is not clear with this drug may harm an unborn baby, it is important to inform the physician if one is pregnant or intending to become pregnant. In addition, studies have indicated that pass into breast milk, thus may affect a nursing infant. As such, it is important to inform the doctor if one is breast-feeding. Children should never be given potassium clavulanate without consulting a physician.
Administration
The drug should be take according to the prescription given by the doctor. Potassium clavulanate is taken orally every 12 hours at the start of a meal to minimize side effects such as stomach upset.
The extended release of the potassium clavulanate tablet must never been chewed or crushed. The medicine should be swallowed as a whole while the chewable tablet must be chewed before swallowing it. Before measuring a dose, the liquid medicine must be shaken well. The medicine should be only used for the full length of prescribed time. It is important not to skip any doses as it may lead to further infection that is resistant to antibiotics.
The medicine should never be taken with or just after ingesting a high-fat meal as it will make it harder for the body to absorb the drug.
Side Effects
Potassium clavulanate may cause diarrhea that is bloody or watery when taken in combination with amoxicillin. It may also lead to severe stomach pain, swelling of the face or tongue, loss of appetite, fever, dark color urine, weakness, or confusion. The medicine may cause jaundice or yellowing of skin as well as vaginal itching or discharge.
Description
Clavulanate is a β-lactamase inhibitor that is effective against Ambler class A β-lactamases (IC50 values range from 12 to 60 nM). While it is not an effective antibiotic by itself, clavulanate is commonly used with other antibiotics that would be inactivated by β-lactamases secreted by bacteria. It is often combined with amoxicillin and other penicillin-based antibiotics.
Chemical Properties
Pale Yellowish Solid
Originator
Clavulanate ,potassium Lek
Uses
Clavulanic acid is a β-lactam antibiotic produced by several species of Streptomyces. The free acid degrades and is isolated and maintained as either the sodium or potassium salt. Clavulanate is a weak antibiotic, but is a potent inhibitor of β-lactamases. In combination with penicillin and cephalosporins, it shows potent synergistic activity. Clavulanic acid is a suicide inhibitor, covalently binding to a serine residue in the active site of the β-lactamase.
Uses
Clavulanic Acid is a β-lactamase inhibitor, typically added to amoxicillin to increase its effectiveness.
Uses
A -Lactamase inhibitor, typically added to amoxicillin to increase its effectiveness
Definition
ChEBI: A potassium salt having clavulanate as the counterion. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes and has only weak anitbiotic activity when administered alone. However it can be used in combination with amoxicillin trihydrate (under the trade name Augmentin) for treatment of a variety of bacterial infections, where it prevents antibiotic inactivation by microbial lactamases.
Manufacturing Process
Clavulanic acid may be obtained by aerobic cultivation of Streptomyces
clavuligerus in conventional nutrient media at, for example, about 25°-30°C
under roughly neutral conditions.
Cultivation of Streptomyces clavuligeru:
Streptomyces clavuligerus was cultivated at 26°C on agar slopes containing
1% Yeatex (yeast extract) ("Yeatex" is a Registered Trade mark), 1% glucose
and 2% Oxoid agar No. 3, pH 6.8. A sterile loop was used to transfer
mycelium and spores from the slope into 100 ml of a liquid medium in a 500
ml Ehrlenmeyer flask. The liquid medium had the following composition: Oxoid
Malt Extract 10g/L, Oxoid Bacteriological Peptone 10g/L, Glycerol 20 g/L, Tap
water 1 L.
The medium was adjusted to pH 7.0 with sodium hydroxide solution and 100
ml volumes dispensed into flasks which were closed with foam plugs prior to
autoclaving at 15 lb/sq.in. for 20 min. An inoculated seed flask was shaken for
3 days at 26°C on a rotary shaker with a 2 inch throw and a speed of 240
r.p.m.
Production stage flasks containing the liquid medium described above were
inoculated with 5% vegetative inoculum and grown under the same conditions
as the seed flask.
Clavulanic acid may be extracted from the culture medium. Normally the cells
of the Streptomyces clavuligerus are first removed from culture medium by
filtration or centrifugation. Then clavulanic acid is extracted into an organic
solvent, for example, n-butanol or ethyl acetate, or n-butyl acetate, or methyl
isobutyl ketone. Then n-butanol fraction are treated with new aqueous phase
using potassium hydrogen carbonate and then this aqueous phase is washed
with n-butanol. This aqueous extract, after separation of the phases, is
concentrated under reduced pressure. Freeze-drying at -20°C may also be
employed to provide a solid crude preparation of the potassium Z-(2R,5R)-3-
(β-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3,2,0]heptane-2-carboxylate
(clavulanate potassium).
Therapeutic Function
Beta-lactamase inhibitor
Potassium clavulanate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
View Lastest Price from Potassium clavulanate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Clavulanate potassium
61177-45-5
|
US $15.00 / kg | 1kg | 99.912% | 10ton | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-22 | Clavulanate Potassium
61177-45-5
|
US $120.00-55.00 / Kg/Bag | 1Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-12 | Potassium Clavulanate
61177-45-5
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd |
-

- Clavulanate potassium
61177-45-5
- US $15.00 / kg
- 99.912%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Clavulanate Potassium
61177-45-5
- US $120.00-55.00 / Kg/Bag
- 0.99
- Sinoway Industrial co., ltd.
-

- Potassium Clavulanate
61177-45-5
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd