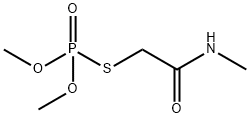
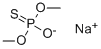Omethoate
- CAS No.
- 1113-02-6
- Chemical Name:
- Omethoate
- Synonyms
- S6876;s-6876;MODERN;B-45432;FOLIMAT;OMETHOAT;ent25,776;P=O-Rogor;bay 45432;OMETHOATE
- CBNumber:
- CB6109938
- Molecular Formula:
- C5H12NO4PS
- Molecular Weight:
- 213.19
- MDL Number:
- MFCD00055441
- MOL File:
- 1113-02-6.mol
| Melting point | -27.9°C |
|---|---|
| Boiling point | 135°C |
| Density | 1.3200 |
| vapor pressure | 3.3×10-3Pa (20 °C) |
| Flash point | 100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| Water Solubility | Miscible |
| pka | 14.40±0.46(Predicted) |
| form | liquid |
| BRN | 1785256 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 1113-02-6(CAS DataBase Reference) |
| EWG's Food Scores | 6-7 |
| FDA UNII | 28U28EWE79 |
| NIST Chemistry Reference | Omethoate(1113-02-6) |
| EPA Substance Registry System | Omethoate (1113-02-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300-H311-H400 |
| Precautionary statements | P264-P270-P273-P280-P301+P310-P302+P352+P312 |
| Hazard Codes | T+;N,N,T+,T |
| Risk Statements | 21-25-50 |
| Safety Statements | 1/2-23-36/37-45-61 |
| RIDADR | 3018 |
| WGK Germany | 3 |
| RTECS | TF8050000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29309090 |
| Hazardous Substances Data | 1113-02-6(Hazardous Substances Data) |
| Toxicity | LD50 oral in rabbit: 50mg/kg |
Omethoate Chemical Properties,Uses,Production
Uses
Omethoate is a systemic insecticide used for the control of (mostly) sucking insects and mites in a wide variety of crops.
Uses
Insecticidal, acaricidal, and fungicidal combinations of carboxamides and other pesticides.
Definition
ChEBI: Omethoate is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide and an agrochemical. It is functionally related to a N-methyl-2-sulfanylacetamide.
Contact allergens
Contact dermatitis from omethoate-dimethoxon is rare.
Metabolic pathway
Omethoate is the P=O analogue (oxon) of dimethoate. Dimethoate is metabolised via oxidative desulfuration to omethoate which is the active anti-acetylcholinesterase metabolite. This biotransformation occurs in all media: hence the metabolic pathways of both compounds have much in common. However, degradative pathways acting directly on dimethoate such as O-demethylation, N-demethylation and hydrolysis of the amide function mean that the balance between activation and degradative metabolism will influence their respective selective toxicities. In mammals there are two main routes of degradation: (i) glutathione transferase mediated O-demethylation (ii) hydrolysis to N-methylthioglycolamide which is S-methylated by S-adenosylmethionine and subsequently thiooxidised. In soil and plants the N-methylthioglycolamide moiety formed from the hydrolysis of omethoate undergoes a complex series of C1, C2 and C3 biotransformations ultimately leading to oxalate and citrate respectively and total degradation to C02. The information reported below is derived largely from an evaluation prepared by the UK MAFF Pesticide Safety Directorate (PSD, 1993).
Metabolism
Orally administered omethoate to rats is rapidly metabolized and excreted in the urine; the main metabolites are O-demethylomethoate and N-methyl-2- methylsulfinylacetamide. O-Demethylation and hydrolysis of the P?S bond are main degradation routes both in mammals and plants. Omethoate is rapidly degraded in soils with DT50 of a few days.
Degradation
Omethoate was slowly hydrolysed in acidic media but more rapidly under alkaline conditions. The half-lives for hydrolysis at pH 4,7, and 9 were 102 days, 17 days and 28 hours, respectively (PM). Omethoate does not absorb light at wavelengths above 250 nm and it is thus unlikely to be subject to photodecomposition. An aqueous solution of omethoate was irradiated for 14 hours with a high pressure filtered mercury vapour lamp without detectable photolysis occurring (PSD, 1993).
Toxicity evaluation
The acute oral LD50 for rats is about 25 mg/kg. Inhalation LC50 (4 h) for rats is 0.3 mg/L air. ADI is 0.3 μg/kg b.w.
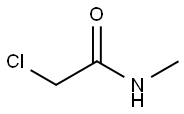
Omethoate Preparation Products And Raw materials
Raw materials
1of4