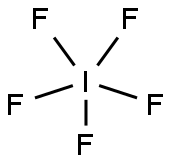
Iodine pentafluoride
- CAS No.
- 7783-66-6
- Chemical Name:
- Iodine pentafluoride
- Synonyms
- IF5;Pentafluoroiodide;Un2495;Iodinefluoride;Fluorine iodide;Iodpentafluorid;Iodine fluoride;Einecs 232-019-7;Pentafluoroiodine;Pentalfluoroiodide
- CBNumber:
- CB8111616
- Molecular Formula:
- F5I
Lewis structure

- Molecular Weight:
- 221.9
- MDL Number:
- MFCD00040534
- MOL File:
- 7783-66-6.mol
- MSDS File:
- SDS
| Melting point | 9.4°C |
|---|---|
| Boiling point | 104.5°C |
| Density | 3,2 g/cm3 |
| vapor pressure | 26.664hPa at 20℃ |
| solubility | reacts with H2O |
| form | yellow liquid |
| color | yellow |
| Water Solubility | reacts violently with H2O [HAW93] |
| Exposure limits | TLV-TWA 2.5 mg(F)/m3 (ACGIH, MSHA, and OSHA). |
| CAS DataBase Reference | 7783-66-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | LFD5C04ER3 |
| NIST Chemistry Reference | Iodine pentafluoride(7783-66-6) |
| EPA Substance Registry System | Iodine pentafluoride (7783-66-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H311-H314-H330-H271-H301 |
| Precautionary statements | P210-P220-P221-P280-P283-P306+P360-P371+P380+P375-P370+P378-P501-P264-P270-P301+P310-P321-P330-P405-P501-P280-P302+P352-P312-P322-P361-P363-P405-P501-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 |
| Hazard Codes | O |
| Risk Statements | 8-14-34 |
| Safety Statements | 7/9-17-36/37/39-45 |
| RIDADR | 2495 |
| Hazard Note | Oxidising agent |
| HazardClass | 5.1 |
| PackingGroup | I |
Iodine pentafluoride price More Price(1)
Iodine pentafluoride Chemical Properties,Uses,Production
Chemical Properties
iodine pentafluoride (IF5) is a colorless to pale yellow fuming liquid with a pungent odor. The highly reactive IF5 (melting point 9.4 °C) is distillable at 100.5 °C without any decomposition. Decomposition commences at temperatures above 400 °C. IF5 is not as popular as p-TolIF2 because F2 gas is used in its preparation and it decomposes in air by moisture emitting corrosive HF.
Iodine pentafluoride has good thermal stability and is the most stable halogen compound. The reactivity of IF5 is much weaker than that of other halogen compounds, and it does not interact with silicon, magnesium, copper, iron, and chromium at room temperature, and only interacts with molybdenum, tungsten, phosphorus, arsenic, antimony, boron, etc. The corresponding fluorides are generated, such as:
2 IF5 + 2 Sb → 2 SbF5 + I2
6 IF5 + 10 B → 10 BF3 + 15 I2
It is the least reactive of the halogen fluorides and classified as a soft fluorinating reagent. It is used as a mildest, selective fluorinating reagent in the fluorination of organic molecules.
Physical properties
Black crystalline solid; exists in two modifications: stable black needlesknown as alpha form that produces ruby-red color in transmitted light, and alabile, metastable beta modification consisting of black platelets which appearbrownish-red in transmitted light; density of alpha form 3.86 g/cm3at 0°C;density of beta form 3.66 g/cm3at 0°C; alpha form melts at 27.3°C, vapor pressure being 28 torr at 25°C; beta form melts at 13.9°C; liquid iodine monochloride has bromine-like reddishbrown color; liquid density 3.10 g/mL at 29°C;viscosity 1.21 centipoise at 35°C; decomposes around 100°C; supercools belowits melting point; polar solvent; as a liquid it dissolves iodine, ammoniumchloride and alkali metal chlorides; liquid ICl also miscible with carbon tetrachloride, acetic acid and bromine; the solid crystals dissolve in ethanol, ether,acetic acid and carbon disulfide; solid ICl also dissolves in conc. HCl butdecomposes in water or dilute HCl.
Uses
Iodine pentafluoride is a fluorinating agent and strong oxidant, which plays an important role in organic synthesis. It can be used to prepare pentafluoroiodoethane:
5 CF2=CF2 + IF5 + 2 I2 → 5 C2F5I
In addition, iodine pentafluoride can also react with isothiocyanato to generate fluorinated hydrocarbon derivatives, and to synthesize other fluorine-containing compounds.
Iodine pentafluoride (IF5) is rarely used to fluoride common organic compounds due to the difficulty in controlling their reactivity.
Preparation
Iodine pentafluoride(IF5) is produced by the action of elemental fluorine on iodine which has a low melting point. The reaction is performed either in solvent or heating iodine to maintain its liquid state. In a commercial preparation about one percent iodine is dissolved in IF5 and brought into fluorination chamber where iodine reacts with fluorine and produces more IF5 which is returned to the receiving vessel. Only a small portion of IF5 is withdrawn from the receiving vessel as product, while the major portion of IF5 is used for dissolving more iodine. This process goes on and on until all iodine is converted into IF5.
In another industrial process, iodine is heated above its melting point (113℃) and reacted with elemental fluorine under three atmosphere of pressure. A precaution is taken not to heat iodine above 150℃ to avoid the formation of another product, IF7. Fluorine is added continuously until all iodine has reacted and converted to IF5.
General Description
Iodine pentafluoride appears as a toxic colorless fuming liquid (m.p. 9° C). Decomposed by water to iodine and hydrofluoric acid. Contact with organic materials may cause their ignition. Corrosive to metals and tissue. Prolonged exposure of the container to fire or heat may result in their violent rupturing and rocketing. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects.
Air & Water Reactions
Fumes in air. Reaction with water or water-containing materials is violent, [Mellor 2, Supp. 1:176, 1956].
Reactivity Profile
A powerful oxidizer. Attack glass. Reacts violently with water or strong bases (potassium hydroxide, sodium hydroxide). Pentalfluoroiodide chars and usually ignites organic matter. Contact with boron, silicon, red phosphorus, sulfur, arsenic, antimony, bismuth, molybdenum and tungsten causes incandescence. Contact with potassium or sodium leads to explosions. Causes aluminum (foil, powder) to ignite. Explosive reactions with tetraiodoethylene, diethylaminotrimethylsilane. Violent reactions wilh benzene, dimethyl sulfoxide, tetraiodoethylene [Bretherick, 5th ed., 1995, p. 1434]. IF5 reacts explosively with diethylaminotrimethylsilane even at low temperature. (Oates, G. et al., J. Chem. Soc., Dalton Trans., 1974, 1383).
Health Hazard
Toxic by ingestion and inhalation, corrosive to skin and mucous membranes. Iodinepentafluoride is highly corrosive. Contactwith the skin can cause severe burns. Thevapors are highly irritating to the skin, eyes,and mucous membranes.
Fire Hazard
Dangerous fire risk, May ignite combustibles (wood, paper, oil, clothing, etc.). React vigorously and/or explosively with water. Produce toxic and/or corrosive substances on contact with water. Flammable/toxic gases may accumulate in tanks and hopper cars. Some may produce flammable hydrogen gas upon contact with metals. Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Non flammable
Purification Methods
The iodine pentafluoride was purified by trap-to-trap distillation over preheated sodium fluoride in order to remove hydrogen fluoride. The iodine pentafluoride was then transferred by vacuum distillation to a vessel containing a dry mercury metal. The mixture was shaken mechanically for 5 minutes at room temperature to remove iodine. Once the mercury metal treatement was finished, iodine pentafluoride was stored over sodium fluoride in a sealed Pyrex glass vessel cooled to -196 °C . The purified iodine pentafluoride was colourless.
Iodine pentafluoride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| BEYOND INDUSTRIES (CHINA) LIMITED | +86-21-52699951; +8613917686115 | sales@beyondindustriesgroup.com | China | 699 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8349 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
View Lastest Price from Iodine pentafluoride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-21 | Iodine pentafluoride
7783-66-6
|
US $0.00 / kg | 10kg | 99.99% | 20mt | Shaanxi Didu New Materials Co. Ltd | |
 |
2019-07-06 | Pentalfluoroiodide
7783-66-6
|
US $1.00 / kg | 1 g | 99% | 100KG | Career Henan Chemical Co |
-

- Iodine pentafluoride
7783-66-6
- US $0.00 / kg
- 99.99%
- Shaanxi Didu New Materials Co. Ltd
-

- Pentalfluoroiodide
7783-66-6
- US $1.00 / kg
- 99%
- Career Henan Chemical Co