DL-リンゴ酸 化学特性,用途語,生産方法
外観
白色の結晶又は結晶性の粉末
定義
本品は、次の化学式で表される有機酸である。
溶解性
水、エタノール及びアセトンに溶けやすく、ジエチルエーテルに溶けにくい。
解説
l -,D-リンゴ酸は遊離の状態,または塩としてリンゴ,ブドウなどの果実中に存在する。潮解性,無色の針状晶。融点 100℃。比旋光度は濃度によって変化し,希薄水溶液たとえば 8.4%濃度では比旋光度は-2.3゜であるが,34%濃度では0°,70%では+3.3°となる。清涼飲料水の酸味に使われる。
用途
酸の1次標準物質としてアルカリ標準液の標定に用いる(当量67.045)。
用途
酸味剤、医薬品原料、乳化安定剤。
用途
食品の酸味料、呈味改良剤
化粧品の成分用途
pH調整剤、香料
使用
Malic acid, HOOCCH(OH).CH2COOH, also known as hydroxysuccinic acid, is a colorless solid. It is soluble in water and alcohol. Malic acid exists in two optically active forms and a racemic mixture. It is used in medicine and found in apples and other fruits.
The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
定義
ChEBI: Malic acid is a 2-hydroxydicarboxylic acid that is succinic acid in which one of the hydrogens attached to a carbon is replaced by a hydroxy group. It has a role as a food acidity regulator and a fundamental metabolite. It is a 2-hydroxydicarboxylic acid and a C4-dicarboxylic acid. It derives from a succinic acid. It is a conjugate acid of a malate(2-) and a malate.
調製方法
Malic acid is manufactured by hydrating maleic and fumaric acids
in the presence of suitable catalysts. The malic acid formed is then
separated from the equilibrium product mixture.
一般的な説明
The chiral resolution of DL-malic acid by ligand-exchange capillary electrophoresis was studied.
化学性质
酸味料,乳化安定剤,加工食品保存剤
使用用途
1. 食品添加物としての用途
リンゴ酸は爽やかな酸味を持っているため、酸味料として飲料水や食品に添加されます。
2. コスメ分野での利用
リンゴ酸には歯垢や歯石の付着予防効果があるため、ホワイトニング効果を期待した歯磨き粉に配合されています。
3. 工業分野での利用
リンゴ酸にはキレート作用があり、金属原子と錯体を形成する能力を有しています。そのため、金属表面の洗浄剤として利用されています。
4. クエン酸回路中間体としての役割
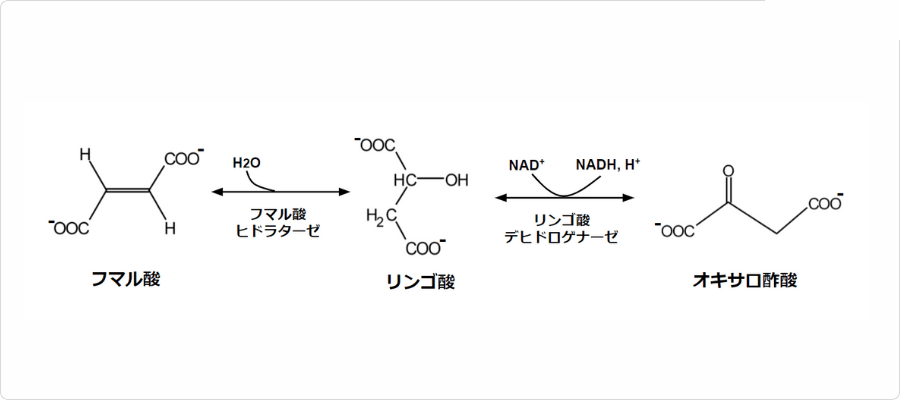
図2. クエン酸回路におけるリンゴ酸の反応
本化合物はクエン酸回路の中間体の一つであり、リンゴ酸デヒドロゲナーゼによってに変換されます。そのため、本化合物は酸素呼吸を行う生物のエネルギー代謝において非常に重要な役割を果たしています。
応用例(製薬)
Malic acid is used in pharmaceutical formulations as a generalpurpose
acidulant. It possesses a slight apple flavor and is used as a
flavoring agent to mask bitter tastes and provide tartness. Malic
acid is also used as an alternative to citric acid in effervescent
powders, mouthwashes, and tooth-cleaning tablets.
In addition, malic acid has chelating and antioxidant properties.
It may be used with butylated hydroxytoluene as a synergist in order
to retard oxidation in vegetable oils. In food products it may be used
in concentrations up to 420 ppm.
Therapeutically, malic acid has been used topically in combination
with benzoic acid and salicylic acid to treat burns, ulcers, and
wounds. It has also been used orally and parenterally, either
intravenously or intramuscularly, in the treatment of liver disorders,
and as a sialagogue.
作用機序
Malic acid is absorbed from the gastrointestinal tract from whence it is transported via the portal circulation to the liver. There are a few enzymes that metabolize malic acid. Malic enzyme catalyzes the oxidative decarboxylation of L-malate to pyruvate with concomitant reduction of the cofactor NAD+ (oxidized form of nicotinamide adenine dinucleotide) or NADP+ (oxidized form of nicotinamide adenine dinucleotide phosphate). These reactions require the divalent cations magnesium or manganese. Three isoforms of malic enzyme have been identified in mammals: a cytosolic NADP+-dependent malic enzyme, a mitochondrial NADP+- dependent malic enzyme and a mitochondrial NAD(P)+-dependent malic enzyme. The latter can use either NAD+ or NADP+ as the cofactor but prefers NAD+. Pyruvate formed from malate can itself be metabolized in a number of ways, including metabolism via a number of metabolic steps to glucose. Malate can also be metabolized to oxaloacetate via the citric acid cycle. The mitochondrial malic enzyme, particularly in brain cells may play a key role in the pyruvate recycling pathway, which utilizes dicarboxylic acids and substrates, such as glutamine, to provide pyruvate to maintain the citric acid cycle activity when glucose and lactate are low.
安全性プロファイル
A poison by
intraperitoneal route. Moderately toxic by
ingestion. A skin and severe eye irritant.
When heated to decomposition it emits
acrid smoke and irritating fumes.
安全性
Malic acid is used in oral, topical, and parenteral pharmaceutical
formulations in addition to food products, and is generally regarded
as a relatively nontoxic and nonirritant material. However,
concentrated solutions may be irritant.
LD
50 (rat, oral): 1.6 g/kg(3)
LD
50 (rat, IP): 0.1 g/kg
貯蔵
Malic acid is stable at temperatures up to 150°C. At temperatures
above 150°C it begins to lose water very slowly to yield fumaric
acid; complete decomposition occurs at about 180°C to give
fumaric acid and maleic anhydride.
Malic acid is readily degraded by many aerobic and anaerobic
microorganisms. Conditions of high humidity and elevated
temperatures should be avoided to prevent caking.
The effects of grinding and humidity on malic acid have also
been investigated.
The bulk material should be stored in a well-closed container, in
a cool, dry place.
合成方法
フマル酸からフマラーゼを用いた酵素法により生産される
純化方法
Crystallise the acid from acetone, then from acetone/CCl4, or from ethyl acetate by adding pet ether (b 60-70o). Dry it at 35o under 1mm pressure to avoid formation of the anhydride. [Beilstein 3 IV 1124.]
不和合性
Malic acid can react with oxidizing materials. Aqueous solutions
are mildly corrosive to carbon steels.
規制状況(Regulatory Status)
GRAS listed. Both the racemic mixture and the levorotatory isomer
are accepted as food additives in Europe. The DL and L forms are
included in the FDA Inactive Ingredients Database (oral preparations).
Included in nonparenteral and parenteral medicines licensed
in the UK. Included in the Canadian List of Acceptable Nonmedicinal
Ingredients.
DL-リンゴ酸 上流と下流の製品情報
原材料
準備製品