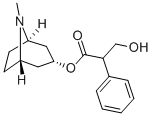
아트로핀
|
|
아트로핀 속성
- 녹는점
- 115-118 °C
- 끓는 점
- 431.53°C (rough estimate)
- 밀도
- 1.0470 (rough estimate)
- 굴절률
- 1.5200 (estimate)
- 인화점
- 2℃
- 저장 조건
- -20°C
- 용해도
- H2O: 2 mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 9.7(at 21℃)
- 색상
- 하얀색
- 수용성
- 1.6g/L(18℃)
- 감도
- Light Sensitive
- Merck
- 14,875
- BRN
- 91260
- InChIKey
- RKUNBYITZUJHSG-SPUOUPEWSA-N
- CAS 데이터베이스
- 51-55-8(CAS DataBase Reference)
- NIST
- Atropine(51-55-8)
안전
- 위험 및 안전 성명
| 위험품 표기 | T+,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/28-36/37/38-20/21/22-36-11 | ||
| 안전지침서 | 25-45-36-26-36/37-16 | ||
| 유엔번호(UN No.) | 1544 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | CK0700000 | ||
| F 고인화성물질 | 8-10-23 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29399900 | ||
| 유해 물질 데이터 | 51-55-8(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 750 mg/kg (Cahen, Tvede) |
아트로핀 C화학적 특성, 용도, 생산
개요
Atropine is considered to be the most effective antidote for both OP and CB intoxication. By effectively competing with acetylcholine for the same cellular receptors, it prevents overstimulation of the autonomous parasympathetic system. Most importantly, it helps prevent asphixia, the main cause of death. In human subjects, it is customary to constantly infuse atropine in order to maintain optimal concentration throughout recovery from the “cholinergic crisis.” In wildlife rehabilitation, this is impractical and subjects need to be repeatedly injected with atropine.화학적 성질
Atropine, also known as daturine, C17H23NO3, white, crystalline substance, optically inactive, but usually contains levorotatory hyoscyamine. Compound is soluble in alcohol, ether, chloroform, and glycerol; slightly soluble in water.물리적 성질
Appearance: atropine appears as colourless, odourless crystals or a white crystalline powder. Solubility: very soluble in water and soluble in ethanol. Melting point: melting point of atropine isn’t higher than 189?°C (melting time decomposition) (Chinese Pharmacopoeia), 114–118?°C (United States Pharmacopeia) and 115– 119?°C (British Pharmacopoeia). The chemical structure of atropine is made up of amino alcohol esters. It is easy for atropine to be hydrolysed into tropine and despun tropic acid under alkaline condition. Atropine is stable in faintly acid and neutral aqueous solution, most stable at pH 3.5–4.0.용도
Atropine is used in medicine and is an antidote for cholinesteraseinhibiting compounds, such as organophosphorus insecticides and certain nerve gases. Atropine is commonly offered as the sulfate. Atropine is used in connection with the treatment of disturbances of cardiac rhythm and conductance, notably in the therapy of sinus bradycardia and sick sinus syndrome. Atropine is also used in some cases of heart block. In particularly high doses, atropine may induce ventricular tachycardia in an ischemic myocardium. Atropine is frequently one of several components in brand name prescription drugs.Indications
This product was recorded in the Pharmacopoeia of the People’s Republic of China (2015), the British Pharmacopoeia (2017), the United States Pharmacopeia (40), the Japanese Pharmacopoeia (17th ed.), the Indian Pharmacopoeia (2010), the European Pharmacopoeia (9.0th ed.), the International Pharmacopoeia (5th ed.) and the Korean Pharmacopoeia (10th ed.). Atropine sulphate is commonly used in clinics. Dosage forms are injection, tablet and eye ointment; atropine sulphate was mainly used to treat toxic shock and organic phosphorus pesticide poisoning, to relieve visceral colic, as preanaesthetic medication and to reduce bronchial mucus secretion. The indications of atropine sulphate eye gel are iridocyclitis, fundus examination and mydriasis.생산 방법
Atropine is prepared by extraction from Datura stramonium, or synthesized. The compound is toxic and allergenic.정의
An alkaloid that is the 3(s)-endo isomer of atropine.World Health Organization (WHO)
Atropine, an alkaloid with anticholinergic activity extracted from Atropa belladonna, has been widely used in medicines for centuries for its antispasmodic and mydriatic properties. It is also used for premedication prior to anaesthesia. Preparations containing atropine remain available and the substance is included in the WHO Model List of Essential Drugs.일반 설명
Atropine is the tropine ester of racemictropic acid and is optically inactive. It possibly occurs naturallyin various Solanaceae, although some claim, with justification,that whatever atropine is isolated from naturalsources results from racemization of (-)-hyoscyamine duringthe isolation process. Conventional methods of alkaloidisolation are used to obtain a crude mixture of atropine andhyoscyamine from the plant material. This crude mixture isracemized to atropine by refluxing in chloroform or by treatmentwith cold dilute alkali. Because the racemizationprocess makes atropine, an official limit is set on thehyoscyamine content by restricting atropine to a maximumlevorotation under specified conditions.Atropine occurs in the form of optically inactive, white,odorless crystals possessing a bitter taste. It is not very solublein water (1:460, 1:90 at 80°C) but is more soluble inalcohol (1:2, 1:1.2 at 60°C). It is soluble in glycerin (1:27),in chloroform (1:1), and in ether (1:25). Saturated aqueoussolutions are alkaline in reaction (pH 9.5). The free baseis useful when nonaqueous solutions are to be made, such asin oily vehicles and ointment bases. Atropine has a plasmahalf-life of about 2 to 3 hours. It is metabolized in the liverto several products, including tropic acid and tropine.
위험도
Extremely toxic, poison, paralyzes the parasympathetic nervous system by blocking the action of acetylcholine at nerve endings.건강위험
The toxic effects are similar to atropine. Thesymptoms at toxic doses are dilation of the pupils, palpitation, blurred vision, irritation,confusion, distorted perceptions, hallucinations,and delirium. However, the mydriaticeffect is stronger than that of many othertropane alkaloids. Scopolamine is about threeand five times more active than hyocyamineand atropine, respectively, in causing dilationof the pupils. Its stimulating effect on thecentral nervous system, however, is weakerthan that of cocaine but greater than thatof atropine. The oral LD50 value in mice iswithin the range of 1200 mg/kg.The histidine reversion–Ames test formutagenicity gave inconclusive results.
Clinical Use
The best known of the muscarinic blocking drugs are the belladonna alkaloids, atropine (Atropine) and scopolamine (Scopolamine).They are tertiary amines that contain an ester linkage. Atropine is a racemic mixture of DL-hyoscyamine, of which only the levorotatory isomer is pharmacologically active.Atropine and scopolamine are parent compounds for several semisynthetic derivatives, and some synthetic compounds with little structural similarity to the belladonna alkaloids are also in use.All of the antimuscarinic compounds are amino alcohol esters with a tertiary amine or quaternary ammonium group.Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion and intramuscular routes: visual field changes, mydriasis @updlary dtlation), and muscle weakness. An experimental teratogen. Other experimental reproductive effects. An alkaloid. When heated to decomposition it emits toxic fumes of NOx.환경귀착
Atropine competitively antagonizes acetylcholine at the neuroreceptor site. Atropine prevents acetylcholine from exhibiting its usual action but does not decrease acetylcholine production. Cardiac muscle, smooth muscle, and the central nervous system are most affected by the antagonism of acetylcholine.Purification Methods
Atropine crystallises from acetone or hot water, and sublimes at ~ 100o/high vacuum. [Beilstein 21/1 V 235.]아트로핀 준비 용품 및 원자재
원자재
준비 용품
아트로핀 공급 업체
글로벌( 259)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 |
bruce@xrdchem.cn | CHINA | 566 | 55 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10248 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 |
sales@czwytech.com | CHINA | 906 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 |
info@konochemical.com | China | 2995 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |