클로로-2-메틸프로판
|
|
클로로-2-메틸프로판 속성
- 녹는점
- -25 °C (lit.)
- 끓는 점
- 51-52 °C (lit.)
- 밀도
- 0.851 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.2 (vs air)
- 증기압
- 4.82 psi ( 20 °C)
- 굴절률
- n
20/D 1.385(lit.)
- 인화점
- −10 °F
- 저장 조건
- Store at <= 20°C.
- 용해도
- 클로로포름에 용해됨
- 물리적 상태
- 액체
- 색상
- 투명한
- 폭발한계
- 1.8-10.1%(V)
- 수용성
- 약간 용해됨
- Merck
- 14,1562
- BRN
- 1730872
- Dielectric constant
- 10.949999999999999
- 안정성
- 안정적인. 가연성이 매우 높습니다. 낮은 인화점을 참고하세요. 강한 산화제와 호환되지 않습니다. 공기보다 밀도가 훨씬 높은 증기는 발화원까지 상당한 거리를 이동할 수 있습니다. 흡습성.
- InChIKey
- NBRKLOOSMBRFMH-UHFFFAOYSA-N
- LogP
- 2.45 at 20-25℃
- CAS 데이터베이스
- 507-20-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11 | ||
| 안전지침서 | 16-29-33-7/9-9 | ||
| 유엔번호(UN No.) | UN 1127 3/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TX5040000 | ||
| 자연 발화 온도 | 570°C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29031980 |
클로로-2-메틸프로판 C화학적 특성, 용도, 생산
화학적 성질
tert-Butyl chloride is a colourless liquid. Miscible with alcohol and ether, insoluble in water. It is flammable and releases toxic phosgene when heated. Reacts violently with oxidizing agents. More toxic than 1-chlorobutane. tert-Butyl Chloride has a wide range of applications in the fields of medicine, pesticides, rubber, plastic additives and other chemical industries.용도
2-Chloro-2-methylpropane is an alkylating agent that is used to functionalize molecules with tert-butyl group.It serves as an effective chlorinating agent, in combination with the ionic liquid, 1-butyl-3-methylimidazolium bromide for converting alcohols into chlorides.
It can also be used to prepare tert-butyl ethers by treating with corresponding alcohols.
주요 응용
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.Safety Profile
Questionable carcinogen with experimental neoplastigenic data. Dangerous fire hazard when exposed to heat, flame (sparks), and oxidlzers. To fight fue, use water, spray, fog, alcohol foam, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC.Synthesis
tert-Butyl chloride is prepared from tertbutyl alcohol (tert-butanol) using an acid catalyzed dehydration reaction.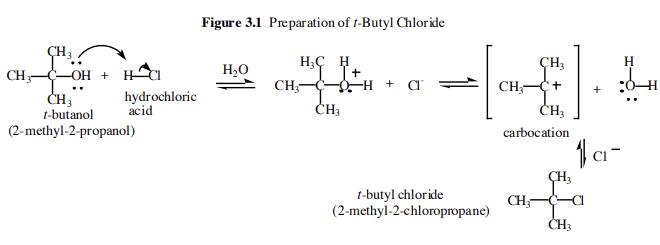
The first step of the overall reaction is an acid-base reaction between the t-butanol and the hydrochloric acid. The t-butanol is a weak base and the hydrochloric acid is a strong acid. The alcoholic oxygen becomes fully protonated and so the equilibrium lies far to the right. In the second step we have the slow loss of water to form a carbocation intermediate. This species is very reactive and is immediately attacked by the chloride ion liberated in the first step to form tert-Butyl chloride.
Purification Methods
Purification methods commonly used for other alkyl halides lead to decomposition. Some impurities can be removed by photochlorination with a small amount of chlorine prior to use. The liquid is washed with ice water, dried with CaCl2 or CaCl2 + CaO and fractionally distilled. It has been further purified by repeated fractional crystallisation by partial freezing. [Beilstein 1 IV 288.]클로로-2-메틸프로판 준비 용품 및 원자재
원자재
준비 용품
1,1'-Bis (di-t-butylphosphino)ferrocene palladium dichloride,
2,6-Di-tert-butylnaphthalene
디 -tert- 부틸 클로로 포스 핀
1,1-DICHLORO-3,3-DIMETHYLBUTANE
2,4,6-트리-터셔리-부틸페놀
트리 -t- 부틸 포스 포늄 테트라 플루오로 보레이트
(R)-(+)-2-Methyl-2-propanesulfinamide
머스크 자일렌
1,1'-Bis(di-tert-butylphosphino)ferrocene
2,2-다이메틸프로판산
1-CHLORO-4-PHENYLBUTANE
4-tert-Butylbenzylamine
ETHOPERMETHRIN,95%
TERT-BUTYLMAGNESIUM CHLORIDE
2,7-DI-TERT-BUTYLNAPHTHALENE
Diclobutrazol
2- 디 -t- 부틸 포스 피노 -2 ', 4', 6'- 트리 -i- 프로필 -1,1'- 비 페닐
부틸(3차)벤젠
Bis(tri-tert-butylphosphine)palladium(0)
테르비나핀
피발로일 클로라이드
tert-부틸나이트라이트
(R)-1-[(1S)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDI-TERT-BUTYLPHOSPHINE
피리다벤
3차-뷰틸아민
트리 -tert- 부틸 포스 핀
클로로-2-메틸프로판 공급 업체
글로벌( 413)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 |
stella@zetchem.com | China | 2141 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |