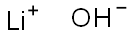하이드록시 리튬
|
|
하이드록시 리튬 속성
- 녹는점
- 470 °C (dec.) (lit.)
- 끓는 점
- 925°C
- 밀도
- 1.43
- 저장 조건
- Store below +30°C.
- 용해도
- 물: 20°C에서 용해성71g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 14[at 20 ℃]
- Specific Gravity
- 2.54
- 색상
- 흰색에서 밝은 노란색
- 냄새
- 냄새 없는
- 수소이온지수(pH)
- 12 (50g/l, H2O, 50℃)
- 수용성
- 113g/L(20℃)
- 감도
- Air Sensitive & Hygroscopic
- Merck
- 14,5534
- 안정성
- 안정적인. 습기와 호환되지 않습니다. 강산, 이산화탄소.
- InChIKey
- WMFOQBRAJBCJND-UHFFFAOYSA-M
- CAS 데이터베이스
- 1310-65-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 52/53-35-20/22-22-34-23 | ||
| 안전지침서 | 45-36/37/39-26-22 | ||
| 유엔번호(UN No.) | 2680 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | OJ6307070 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28252010 | ||
| 독성 | LD50 orally in Rabbit: 210 mg/kg | ||
| 기존화학 물질 | KE-22570 |
하이드록시 리튬 C화학적 특성, 용도, 생산
화학적 성질
lithium hydroxide (LiOH) is a white solid made industrially as the monohydrate (LiOH.H2O) by reacting lime with a lithium ore or with a salt made from the ore. Lithium hydroxide has a closer resemblance to the group 2 hydroxides than to the group 1 hydroxides.물리적 성질
White tetragonal crystals; refractive index 1.464; density 1.46 g/cm3; melts at 450°C; decomposes at 924°C; dissolves in water (12.8g/100g at 20°C and 17.5 g/100g at 100°C); slightly soluble in alcohol. The monohydrate is white monoclinic crystalline solid; refractive index 1.460; density 1.51 g/cm3; soluble in water, more soluble than the anhydrous salt (22.3g and 26.8g/100g at 10 and 100°C, respectively); slightly soluble in alcohol; insoluble in ether.용도
Lithium hydroxide is used in storage batteries and soaps and as CO2 absorber in spacecrafts.정의
A white crystallinesolid, LiOH, soluble in water,slightly soluble in ethanol and insolublein ether. It is known as the monohydrate(monoclinic; r.d. 1.51) and inthe anhydrous form (tetragonal, r.d.1.46; m.p. 450°C; decomposes at924°C). The compound is made by reacting lime with lithium salts orlithium ores. Lithium hydroxide isbasic but has a closer resemblance togroup 2 hydroxides than to the othergroup 1 hydroxides (an example ofthe first member of a periodic grouphaving atypical properties).일반 설명
A clear to water-white liquid which may have a pungent odor. Contact may cause severe irritation to skin, eyes, and mucous membranes. Lithium hydroxide may be toxic by ingestion, inhalation and skin absorption. Lithium hydroxide is used to make other chemicals.공기와 물의 반응
Dilution with water may generate enough heat to cause steaming or spattering.반응 프로필
LITHIUM HYDROXIDE SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Safety Profile
Poison by ingestion and subcutaneous routes. Mtldly toxic by inhalation. A corrosive. When heated to decomposition it emits toxic fumes of Li.Purification Methods
It crystallises from hot water (3mL/g) as the monohydrate. It is dehydrated at 150o in a stream of CO2-free air. It sublimes at 220o with partial decomposition [Cohen Inorg Synth V 3 1957, Bravo Inorg Synth VII 1 1963].하이드록시 리튬 준비 용품 및 원자재
원자재
준비 용품
L-LACTIC ACID LITHIUM SALT
5-Bromoindole-2-carboxylic acid
아세트산리튬
과산화리튬
LITHIUM LACTATE
디리튬 메타실리케이트
4-[4-(Aminomethyl)pyridin-2-yl]piperazine, N1-BOC protected
5-BROMO-1-BENZOFURAN-2-CARBOXYLIC ACID
3-(2-FURYL)PROPANOIC ACID
황산 리튬
Lithium acetate dihydrate
브롬화리튬
6-BROMO-1-BENZOFURAN-2-CARBOXYLIC ACID
5-Chloroindole-2-carboxylic acid
5-METHYLISOXAZOLE-3-CARBONYL CHLORIDE
2,5-Dihydroxyterephthalic acid
리튬 크로뮴산
lithium base grease
질산리튬
1,3,5-TRIMETHYL-1,3,5-TRIPHENYLCYCLOTRISILOXANE
Molybdenum disulfide lithium-based grease
3-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
염화 리튬
하이드록시 리튬 공급 업체
글로벌( 424)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 |
hjt@hong-jin.com | China | 228 | 58 |
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 |
1715438216@qq.com | China | 52 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5964 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1532 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |