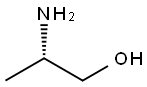
L-알라닌올
|
|
L-알라닌올 속성
- 녹는점
- -2°C
- 끓는 점
- 72-73 °C11 mm Hg(lit.)
- 알파
- 21.8 º (c=2,ethanol)
- 밀도
- 0.965 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.450
- 인화점
- 145 °F
- 저장 조건
- Keep in dark place,Inert atmosphere,2-8°C
- 수용성
- Completely miscible in water
- 용해도
- 에 용해됨클로로포름, 메탄올 (약간 용해됨)
- 물리적 상태
- 기름진 액체
- 산도 계수 (pKa)
- 12.88±0.10(Predicted)
- Specific Gravity
- 0.96
- 색상
- 무색~황색으로 투명함
- optical activity
- [α]20/D +18°, neat
- 감도
- Hygroscopic
- BRN
- 1718865
- CAS 데이터베이스
- 2749-11-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-36/37/38 | ||
| 안전지침서 | 26-36/37/39-45-36 | ||
| 유엔번호(UN No.) | UN 2735 8/PG 2 | ||
| WGK 독일 | 3 | ||
| F 고인화성물질 | 10-23 | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 29221990 |
L-알라닌올 C화학적 특성, 용도, 생산
화학적 성질
Colorless liquid용도
S-(+)-2-Amino-1-propanol is an aliphatic amino alcohol shown to induce an antiproliferative effect in B16 melanoma cells. S-(+)-2-Amino-1-propanol is used in the preparation of oxazolines which are of ten used as ligands in homogeneous catalysis.정의
ChEBI: An amino alcohol that is L-alanine in which the carboxy group has been reduced to the corresponding alcohol.Purification Methods
Purify it as for S-2-amino-3-methylbutan-1-ol below. [Beilstein 4 IV 1615.]L-알라닌올 준비 용품 및 원자재
원자재
준비 용품
1-AdaMantanethylaMine
ERGONOVINE
Oxazole, 2,2'-(1-methylethylidene)bis[4,5-dihydro-4-methyl-, (4S,4'S)-
N-벤질-L-알라니놀
Carbamic acid, N-[(1S)-2-hydroxy-1-methylethyl]-, methyl ester
(S)-2-Amino-tert-butyldimethylsilyloxypropane
(S)-(+)-2-(DIBENZYLAMINO)-1-PROPANOL
(S)-2-메틸-1-(4-니트로벤젠술포닐)아지리딘
(S)-2-((2,6-dichloropyrimidin-4-yl)amino)propan-1-ol
(S)-잔토안트라필
L-알라닌올 공급 업체
글로벌( 480)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Zhejiang Brunova Technology Co., Ltd. | 8617857108485 17857108485 |
info@zjbrunova.com | China | 41 | 58 |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 |
daisy@enlaibio.com | China | 1106 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 |
sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18629 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9354 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32836 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Win-Win Chemical CO., Limited | 0086-577-64498589 |
sales@win-winchemical.com | CHINA | 998 | 58 |