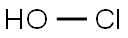하이포클로로스애씨드
|
|
하이포클로로스애씨드 속성
- 끓는 점
- 125-130 °C(Press: 11 Torr)
- 밀도
- 1.1655 g/cm3
- 용해도
- soluble in H2O
- 산도 계수 (pKa)
- pK (25°) 7.49
- 물리적 상태
- 용액에서만 안정함
- 색상
- green-yellow; stable only in aqueous solution
- LogP
- 0.710 (est)
안전
| 유엔번호(UN No.) | 3139 | ||
|---|---|---|---|
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| 기존화학 물질 | KE-20928 |
하이포클로로스애씨드 C화학적 특성, 용도, 생산
물성
수용액으로만 존재하며, 빛이나 열에 의해 상당히 불안정하게 되어 발생기 산소를 발생시킨다.진한 용액은 담황색을 띠며, 자극적인 냄새가 난다.용도
이 산소 라디칼에 의해 강한 산화성을 가지며, 용액 자체나 그 염은 표백·살균제, 소독제로 쓰인다.개요
Hypochlorous acid [7790-92-3], HOCl, Mr 52.5, is only moderately stable in aqueous solution. It is colorless when dilute and yellowish at higher concentrations. Hypochlorous acid is one of the most powerful oxidizing agents known. Hypochlorous acid solution decomposes exothermically. The main decomposition products are hydrochloric acid and oxygen.화학적 성질
Greenish-yellow aqueous solution. Highly unstable, weak acid. Decomposes to hydrogen chloride and oxygen. Can exist only in dilute solutions.물리적 성질
Greenish-yellow aqueous solution; unstable; weak acid, pKa7.40 at 25°C;soluble in water.용도
Disinfectant. Because of its instability, hypochlorous acid is not used extensively as an oxidizing or bleaching agent. It was used mainly in the water treatment industry for slime control, for treatment of drinking water, and for sterilization of swimming pools. These applications have now been taken over by the more stable hypochlorites.정의
ChEBI: Hypochlorous acid is a chlorine oxoacid with formula HOCl; a weak, unstable acid, it is the active form of chlorine in water. It has a role as a human metabolite, an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an EC 2.5.1.18 (glutathione transferase) inhibitor. It is a member of reactive oxygen species and a chlorine oxoacid. It is a conjugate acid of a hypochlorite.제조 방법
Hypochlorous acid is obtained by dissolving chlorine in water, or by addingbleaching powder or sodium hypochlorite to water. A better method of pro-duction is passing chlorine gas into a well-agitated suspension of mercuricoxide:2Cl2 + 2HgO + H2O → HgO•HgCl2 + 2HOCl
or by distilling chlorine hexahydrate and mercuric oxide at low pressure:
2Cl2•6H2O + HgO → 2HOCl + HgCl2 + 5H2O
The latter process can yield a 25% acid solution.
Hypochlorous acid also may be obtained by hydrolysis of chlorine monoflu-oride:
ClF + H2O → H+ + F¯ + HOCl
The above reaction may be explosive and is not recommended for preparinghypochlorous acid.
위험도
Irritant to skin and eyes.하이포클로로스애씨드 준비 용품 및 원자재
원자재
준비 용품
말라카이트그린(옥살산염)
N,N-디메틸하이드라진
VAT 옐로우 4
19-CARBOXYANDROST-4-ENE-3,17-DIONE
2-Chloro-1-propanol
5-Chloro-3,6-dihydroxy-5-androstan-17-one 3-acetate
푸란-2-카르복실산
5-CHLORO-6 B,19-EPOXY-5A -ANDROTANE-3,17-DIONE
2-CHLOROCYCLOHEXANOL
1,2-부틸렌옥사이드
2-CHLORO-5-METHYLPHENOL
에틸렌 클로로히드린
N,N-DIISOPROPYLBENZOTHIAZOLE-2-SULFENAMIDE
1-클로로-2-프로탄올
N-클로로숙신이미드
디클로로(1,3-)-2-프로판올
에피클로로하이드린
N-tert-부틸-2-벤조티아조릴 설펜아미드
산화프로필렌
하이포클로로스애씨드 공급 업체
글로벌( 59)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Wuhan Monad Medicine Tech Co.,LTD | 02768782018 18771942761 |
sales01@whmonad.com | CHINA | 992 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Hebei Qige Biological Technology Co. Ltd | +86 +8618733132031 |
CHINA | 1313 | 58 | |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 |
mack@tongyon.com | China | 2996 | 58 |