| | Pentaborane Basic information |
| Product Name: | Pentaborane | | Synonyms: | (2,3-Muh),(2,5-muh),(3,4-muh),(4,5-muh)-nido-pentaborane(9);2,3:2,5:3,4:4,5-Tetra-muh-nido-pentaborane(9);Chebi:33591;Nido-pentaborane(9);Pentaborane;pentaborane(9) | | CAS: | 19624-22-7 | | MF: | B5H9 | | MW: | 55.06 | | EINECS: | 243-194-4 | | Product Categories: | | | Mol File: | 19624-22-7.mol | 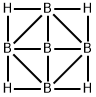 |
| | Pentaborane Chemical Properties |
| Melting point | -46.6° | | Boiling point | bp 60° | | density | d40 0.61 | | vapor density | 2.2 | | solubility | reacts with hot H2O | | form | flammable liquid | | color | Colorless gas or liquid | | Odor | bad odor | | Water Solubility | hydrolyzed if heated [COT88] | | Dielectric constant | 21.0(Ambient) | | CAS DataBase Reference | 19624-22-7 | | EPA Substance Registry System | Pentaborane (19624-22-7) |
| RIDADR | 1380 | | OEB | E | | OEL | TWA: 0.005 ppm (0.01 mg/m3), STEL: 0.015 ppm (0.03 mg/m3) | | HazardClass | 4.2 | | PackingGroup | I | | Hazardous Substances Data | 19624-22-7(Hazardous Substances Data) | | Toxicity | LC50 ihl-rat: 6 ppm/4H AIHAAP 19,46,58 | | IDLA | 1 ppm |
| | Pentaborane Usage And Synthesis |
| Description | Pentaborane is a nonmetallic, colorless liquid with a pungent odor. It decomposes at 300°F (148°C), if it has not already ignited, and will ignite spontaneously in air if impure. It is a dangerous fire and explosion risk, with a flammable range of 0.46%–98% in air. Boiling point is 145°F (64°C), flash point is 86°F (30°C), and ignition temperature is 95°F (35°C), which is extremely low. Any object that is 95°F (35°C) or above can be an ignition source. Ignition sources can be ordinary objects on a hot day in the summer, such as the pavement, metal on vehicles, and even the air. In addition to extreme flammability, it is also toxic by ingestion or inhalation and is a strong irritant. TLV is 0.005 ppm in air, and it is immiscible in water. The four-digit UN identification number is 1380. The NFPA 704 designation for pentaborane is health 4, flammability 4, and reactivity 2. The primary uses are as fuel for air-breathing engines and as a propellant. | | Chemical Properties | Pentaborane is a colorless, volatile liquid.
Unpleasant, sweetish odor, like sour milk. The Odor
Threshold is 0.8 ppm. | | Uses | There appears to be no commercial market for pentaborane.
In the 1950s it was explored as a potential rocket fuel. | | Uses | Reducing agent in propellant fuels | | Preparation | Pentaborane is obtained by passing diborane through a hot tube. Careful control of temperature, pressure, and flow are required to obtain
good yields and avoid further pyrolysis to higher
hydrides. | | General Description | A clear colorless liquid with a pungent odor like sour milk, and flammable.It is corrosive to natural rubber, some synthetic rubber, some greases, and some lubricants and gives off irritating or toxic fumes (or gases) in a fire. It reacts violently with fire. Pentaboron nonahydride incompatible with strong oxidants such as chromium anhydride, chlorate and potassium permanganate, and other contacts. Vapors toxic both under prolonged exposure to low concentrations and short exposure to high concentrations. Density 0.61 g / cm3. Under prolonged exposure to intense heat the containers may rupture violently and rocket. | | Air & Water Reactions | Highly flammable. May ignite spontaneously in air [Merck 11th ed. 1989]. Slowly decomposes in water. | | Reactivity Profile | Pentaborane is an extremely reactive reducing agent. Can ignite spontaneously in contact with air and many other materials. Reactions with oxygen are often violently explosive. Reacts with ammonia to form a diammoniate. Is stabilized by the formation of complexes with N, O, P, or S. Is stable in hydrocarbon solvents, but forms shock sensitive solutions in most carbonyl containing solvents. | | Health Hazard | May cause death or permanent injury after very short exposure to small quantities. | | Fire Hazard | Ignites spontaneously in air. Reacts violently with halogenated extinguishing agents. Boron hydrides present considerable fire and explosion hazard. They undergo explosive reaction with most oxidizing agents, including halogenated hydrocarbons. Fires tend to reignite. On decomposition, Pentaborane emits toxic fumes and can react vigorously with oxidizing materials. Avoid dimethyl sulfoxide, water, most oxidizing agents (including halogenated hydrocarbons). Avoid direct sunlight and sources of ignition, decomposes very slowly at 302. Hazardous polymerization may not occur. | | Safety Profile | Poison by inhalation and intraperitoneal routes. Dangerous fire hazard by chemical reaction; spontaneously flammable in air. Dangerous explosion hazard. To fight fire, use special fire-fighting materials; water is not effective; reacts violently with halogenated extinguishing agents. Get instructions from supplier. Explosive reaction with oxygen. Forms shock-sensitive solutions in solvents containing carbonyl, ether, or ester functions; or halogens. Incompatible with dimethyl sulfoxide. Upon decomposition it emits toxic fumes of B. See also BORANES and BORON COMPOUNDS | | Potential Exposure | Pentaborane is used in rocket propellants
and in gasoline additives. | | Shipping | UN1380 Pentaborane, Hazard Class: 4.2; Labels:
4.2-Spontaneously combustible material, 6.1-Poisonous
materials. Inhalation Hazard Zone A. | | Incompatibilities | Pentaborane is an extremely reactive
reducing agent. It can ignite spontaneously in contact with
air and many other materials. Reactions with oxygen are
often violently explosive. Reacts with ammonia to form
a diammoniate. Reacts on contact with water, oxidizers,
halogens, including halogenated hydrocarbons. May sel-heat
and ignite spontaneously in moist air, decomposes @ 150�C.
Hydrolyzes slowly with heat in water to form boric acid.
Contact with solvents, such as ketones, ethers and esters form
shock-sensitive compounds. Pentaborane is stable in hydrocarbon
solvents, but forms shock sensitive solutions in most
carbonyl containing solvents. Corrosive to natural rubber,
some synthetic rubbers and to some lubricants. Avoid
dimethyl sulfoxide, direct sunlight and sources of ignition. | | Waste Disposal | Incineration with aqueous
scrubbing of exhaust gases to remove B2O3 particulates. |
| | Pentaborane Preparation Products And Raw materials |
|