- Basic Red 9
-

- $0.00 / 1kg
-
2023-06-10
- CAS:569-61-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500000kg
- Basic Red 9
-

- $2.00 / 1KG
-
2019-07-06
- CAS:569-61-9
- Min. Order: 1KG
- Purity: Dye content 70%
- Supply Ability: customise
|
| | Basic Red 9 Basic information |
| Product Name: | Basic Red 9 | | Synonyms: | 4-((4-aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl)-benzenaminm;4,4’-((4-imino-2,5-cyclohexadien-1-ylidene)methylene)dianilinemonohydrochlor;4,4’-((4-imino-2,5-cyclohexadien-1-ylidene)methylene)dianilinemonohydrochlorid;4,4’,4"-triaminotriphenylmethanehydrochloride;4,4’,4"-triaminotriphenylmethan-hydrochlorid;4-[(4-aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-benzenaminm;alpha-(p-aminophenyl)-alpha-(4-imino-2,5-cyclohexadien-1-ylidene)-4-toluidin;basicparafuchsine | | CAS: | 569-61-9 | | MF: | C19H17N3.ClH | | MW: | 323.82 | | EINECS: | 209-321-2 | | Product Categories: | Triphenylmethane;Dyes and Pigments;Stains and DyesDerivatization Reagents TLC;Steroids, Terpenes, Lipids, Bile acids;Analytical Reagents;Microscopy Reagents;TLC Visualization Reagents (by application);Stains and Dyes | | Mol File: | 569-61-9.mol | 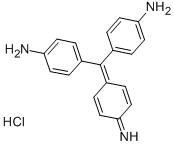 |
| | Basic Red 9 Chemical Properties |
| Melting point | 250 °C | | Boiling point | 479.57°C (rough estimate) | | density | 0.999 g/mL at 20 °C | | refractive index | n20/D 1.334 | | Fp | 11 °C | | storage temp. | Store at RT. | | solubility | ethanol: soluble1mg/mL | | Colour Index | 42500 | | form | Solid | | color | metallic green | | PH Range | 1.0 - 3.1, purple to red | | Water Solubility | 10 g/L (25 ºC) | | λmax | 545 nm | | BRN | 4164603 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | Biological Applications | Detecting breast cancer; treating pathogens | | InChIKey | JUQPZRLQQYSMEQ-UHFFFAOYSA-N | | CAS DataBase Reference | 569-61-9 | | IARC | 2B (Vol. 57, 99) 2010 | | EPA Substance Registry System | C.I. Basic Red 9, monohydrochloride (569-61-9) |
| | Basic Red 9 Usage And Synthesis |
| Chemical Properties | green crystalline powder | | Chemical Properties | C.I. Basic red 9 is a colorless to red crystalline
solid or green powder. | | Uses | Pararosaniline Hydrochloride is a red dye used for textiles. Dyes and metabolites, Environmental Testing. | | Uses | Antischistosoma. | | Definition | ChEBI: A hydrochloride that is the monohydrochloride of 4,4'-[(4-iminocyclohexa-2,5-dien-1-ylidene)methanediyl]dianiline. One of the major constituents of Basic fuchsin, together with rosanilin, magenta II and new fuchsin. | | Preparation | commonly known as (Para Magenta, Para Rosaniline) (a) 4-(4-Aminobenzyl)benzenamine?and aniline, Aniline hydrochloride, the difficulties and ferric chloride in 170 ℃ heating hours;?(b) aniline,p-Methylaniline and its hydrochloride and iron, ferrous chloride or 1-Nitrobenzene?heating; (C) aniline?and p-Methylaniline?was treated with arsenic oxide; (d) aniline?tetrachloride carbon heat. | | General Description | Colorless to red crystals or green powder. | | Air & Water Reactions | Insoluble in water. | | Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition Basic Red 9 emits very toxic fumes of hydrogen chloride and nitrogen oxides. | | Fire Hazard | Flash point data for Basic Red 9 are not available; however, Basic Red 9 is probably combustible. | | Safety Profile | Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Mildly toxic by ingestion. Mutation data reported. When heated to decomposition it emits very toxic fumes of HCl and NOx. | | Potential Exposure | Used as a dye for textiles, paper;
printing, computer and photo imaging inks, leather, and
many consumer products; as a microbiological/microscopy
stain for bacilli, including tubercle and influenza. | | Carcinogenicity | Basic red 9 monohydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity fromstudies in experimental animals. | | Shipping | UN 3143 Dyes, solid, toxic, n.o.s., or dye intermediates,
solid, toxic, n.o.s., Hazard Class: 6.1; Labels:
6.1—Poisonous materials, Technical Name Required. | | Properties and Applications | colourful red blue light. Slightly soluble in cold water, soluble in hot water for red, easily soluble in ethanol is cherry red. The strong sulfuric acid for yellow brown, after dilute for purple. Used in the manufacture of blue ink, also can dye tannins mordant dyeing cotton.
|
Standard( Cotton )
|
Light Fastness
|
Persperation Fastness
|
Ironing Fastness
|
Soaping
|
|
Fading
|
Stain
|
Fading
|
Stain
|
Fading
|
Stain
|
|
A
|
2
|
1
|
|
|
|
1
|
|
| | Incompatibilities | May be combustible; powder or liquid
may form explosive mixture with air. Incompatible with
oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides. | | Waste Disposal | Use a licensed professional
waste disposal service to dispose of this material. Dissolve
or mix the material with a combustible solvent and burn in
a chemical incinerator equipped with an afterburner and
scrubber. All federal, state, and local environmental regulations
must be observed. |
| | Basic Red 9 Preparation Products And Raw materials |
|