- Oseltamivir Phosphate
-

- $0.00 / 1kg
-
2024-09-19
- CAS:204255-11-8
- Min. Order: 1kg
- Purity: 99.0%min. HPLC
- Supply Ability: 1000kg
- Oseltamivir Phosphate
-

- $0.00 / 25KG
-
2024-09-19
- CAS:204255-11-8
- Min. Order: 2KG
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Oseltamivir phosphate
-

- $50.60 / 1KG
-
2024-09-19
- CAS:204255-11-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000kg
|
| | Oseltamivir phosphate Chemical Properties |
| Melting point | 196-198°C | | storage temp. | 2-8°C | | solubility | H2O: soluble30mg/mL, clear | | form | powder | | color | white to beige | | optical activity | [α]/D -26 to -36°, c = 1 in H2O | | Water Solubility | Soluble in water (75 mM) | | BCS Class | 1 (CLogP), 3
(LogP) | | InChI | InChI=1/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/s3 | | InChIKey | PGZUMBJQJWIWGJ-IFAKAUOZSA-N | | SMILES | [C@@H]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(=O)C)C(=O)OCC.OP(O)(O)=O |&1:0,10,12,r| | | CAS DataBase Reference | 204255-11-8(CAS DataBase Reference) |
| HazardClass | IRRITANT | | HS Code | 2924299500 |
| | Oseltamivir phosphate Usage And Synthesis |
| Anti-influenza virus | Oseltamivir phosphate is a kind of anti-influenza drugs, under the trade name Tamiflu. Its appearance exhibits as white to yellowish-white powder. It can be divided into type A (alpha) and B (Beta) selective inhibitor of the influenza virus neuraminidase and can prevent the release of virus from infected cells, through inhibiting the activity of the influenza virus neuraminidase, thus achieving the purpose of control of influenza symptoms. Clinically it can be used for treatment of the type A influenza and type B influenza in adults and children of 1 year old or over 1 year old as well as the clinical prevention of influenza A and B in adult and adolescent of 13 year old or over 13 years old.
Oseltamivir phosphate can subject to rapid metabolism into oselatamivr carboxylate after gastrointestinal absorption with 75% of the total oral administrated dose participating in the systemic circulation in the from of oselatamivr carboxylate. The active ingredient of this drug is a potent and unique neuraminidase inhibitors acting on all the processes of influenza virus infection, preventing the replication of all clinically relevant influenza virus strains A or B strain. It has obvious inhibitory effect even in nanomolar concentration in vitro. It has ben observed in vitro that the active metabolite can inhibit the growth of influenza virus as well as in vivo that it can inhibit the replication and pathogenicity of influenza virus. This product, through inhibiting the release of virus from infected cells, can reduce the spread of influenza A or B virus.
The above information is edited by the chemicalbook of Dai Xiongfeng. | | Uses | Oseltamivir Phosphate is an orally active inhibitor of influenza virus neuraminidase; converted in vivo to the active acid metabolite. An antiviral drug. It is a COVID19-related research product. | | Description | Oseltamivir phosphate (Tamiflu) was launched in the US and Switzerland for the treatment of influenza infections by all common strain viruses. It is an oral anti-viral drug approved for the treatment of acute, uncomplicated influenza in patients 2 weeks of age and older whose flu symptoms have not lasted more than two days. This product is approved to treat Type A and B influenza; however, the majority of patients included in the studies were infected with type A, the most common in the U.S. Efficacy of Tamiflu in the treatment of influenza in subjects with chronic cardiac disease and/or respiratory disease has not been established. | | Chemical Properties | White Cyrstalline Solid. It is freely soluble in water. | | Originator | Gilead (US) | | Uses | Oseltamivir phosphate (Tamiflu) is a competitive neuraminidase inhibitor. The prodrug oseltamivir phosphate (Tamiflu) is itself not virally effective; however, once in the liver, it is converted by natural chemical processes, hydrolysed hepatically to its | | Definition | ChEBI: Oseltamivir phosphate is a phosphate salt. It contains an oseltamivir. It is an acetamido cyclohexene that is a structural homolog of SIALIC ACID and inhibits NEURAMINIDASE. | | Preparation | Oseltamivir phosphate (Tamiflu) has been synthesized from cis-2,3-bis(hydroxymethyl)aziridine. After protection of the cis-2,3-bis(hydroxymethyl)aziridine with a Boc group, desymmetrization provided a chiral aziridine, which was a key intermediate to install the required stereogenic center containing a nitrogen atom. Allylation and ring closing metathesis are the key reactions to obtain the cyclic product that was successfully converted to the desired oseltamivir phosphate. DOI: 10.1021/jo3015853
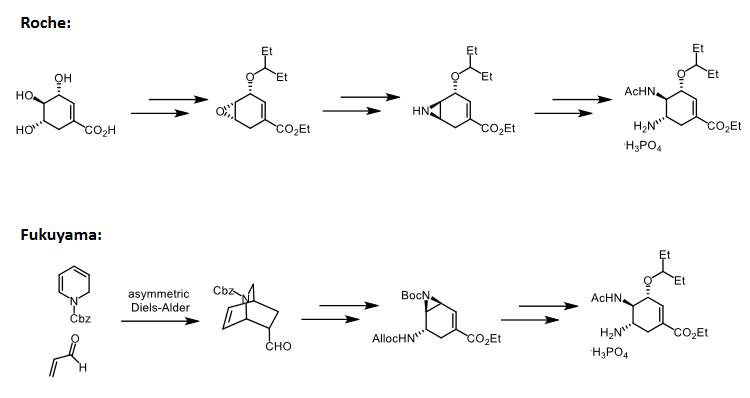
It can also be obtained by a novel 12-step synthesis from (-)-quinic acid. | | Brand name | Tamiflu (Roche). | | Therapeutic Function | Antiviral | | General Description | receptor site showed clearly that additional binding sitesexist for the C-5 acetamido carbonyl group and the arginineresidue at position 152 of the receptor site. In addition, the C-2 carboxyl group of sialic acid binds to Arg 118, Arg 292, andArg 371. Position C-6 is capable of undergoing a hydrophobicinteraction with various amino acids, including Glu, Ala,Arg, and Ile. Maximum binding to neuraminidase occurswhen the C-6 substituent is substituted with a nonpolar chain.In oseltamivir, this nonpolar group is 3-pentyl. An importantfeature of oseltamivir is the ethyl ester, which makes the drugorally efficacious. This drug is the first orally active agent foruse against influenza A and B. It is also indicated for the treatmentof acute illness. If administered within 2 days after theonset of influenza symptoms, the drug is effective. | | Biochem/physiol Actions | Oseltamivir phosphate is an influenza viral neuraminidase inhititor. Oseltamivir phosphate, an antiviral, is used clinically to treat influenza A and influenza B, and to prevent flu after exposure. Oseltamivir phosphate is hydrolyzed in the liver to its active form, oseltamivir carboxylate, which is an inhibitor of influenza viral neuraminidases essential for viral replication. Oseltamivir has a broad spectrum of activity against a range of influenza A and B subtypes with IC50 values for neuraminidases measured from less than 1 nM to approximately 30 nM, depending on the virus subtype. | | Clinical Use | Oseltamivir was approved as the first orally administered neuraminidase inhibitor used against influenza A and B viruses. The drug is indicated for the treatment of uncomplicated acute illness caused by influenza infection. | | Side effects | Side effects with oseltamivir are minor, consist of nausea and vomiting, and occur primarily in the first two days of therapy. | | Veterinary Drugs and Treatments | Although, there is no research published (at the time of writing—
January 2007) documenting oseltamivir safety or efficacy in dogs
or cats, there is much interest and discussion regarding its potential
for the adjunctive treatment of parvovirus infections in dogs.
It may be of benefit for adjunctive treatment of other viral infections,
particularly those with associated secondary bacterial components,
but research or experience is lacking. A recent study performed
in horses, experimentally infected with equine influenza A
(H3N8), documented some efficacy in the attenuation of clinical
signs (pyrexia), viral shedding, and secondary bacterial pneumonias
(Yamanaka, Tsujimura et al. 2006).
Because oseltamivir is the primary antiviral agent proposed for
treatment or prophylaxis for an H5N1 influenza (“bird flu”) pandemic
in humans, its use in veterinary patients is controversial, particularly
due to concerns of adequate drug supply for the human
population and the potential for influenza virus resistance development.
In 2006, the FDA banned the extra-label use of oseltamivir
and other influenza antivirals in chickens, turkeys and ducks. At
the time of writing, its use is still allowed in mammal veterinary
patients, but veterinarians should use the drug prudently and be
cognizant of these public health concerns. | | Metabolism | Oseltamivir is readily absorbed from the GI tract following oral administration. It is a prodrug that is extensively metabolized in the liver, undergoing ester hydrolysis to the active carboxylic acid. Two oxidative metabolites also have been isolated, with the major oxidation product being the ω-carboxylic acid. | | storage | -20°C | | Clinical claims and research | Oseltamivir is the ethyl ester prodrug of GS-4071, the corresponding acid, which is one of the most potent inhibitors of both influenza A and B virus neuraminidase (sialidase) isoenzymes; these glycoproteins are expressed on the virion surface and are essential for virus replication for both A and B strains. Oseltamivir emerged as one of the first two neuraminidase inhibitors to reach the market. GS-4071 demonstrated a low (< 5%) oral bioavailability in animals due to a poor absorption from the gastrointestinal barrier; by incorporating a more lipophilic ester group, the oral bioavailabilty can reach 30 to 100% in mice, rats and dogs. Following oral administration of Oseltamivir in rats, a similar concentration of GS-4071 was found in the bronchoalveolar lining fluid and the plasma which indicated a good penetration of the active compound into the lower respiratory tract. In mice, chickens and ferrets, orally administered Oseltamivir was found to have significant inhibitory effects on A and B influenza infections in protecting against a lethal challenge of virus and lessening virus titer in the lungs or nasal washings. In several clinical trials with patients receiving oral capsules daily, Oseltamivir was shown to be effective in reducing significantly the duration and severity of the clinical symptoms, including fever, cough and general malaise, in both early treatment and prevention. | | Mode of action | Oseltamivir Phosphate is the phosphate salt of oseltamivir, a synthetic derivative prodrug of ethyl ester with antiviral activity. By blocking neuraminidases on the surfaces of influenza viruses, oseltamivir interferes with host cell release of complete viral particles. |
| | Oseltamivir phosphate Preparation Products And Raw materials |
|