- Vonoprazan
-
- $0.00 / 1KG Ton
-
2023-09-08
- CAS:881681-00-1
- Min. Order: 1KG Ton
- Purity: 99%+
- Supply Ability: 2 tons/month
- Vonoprazan
-
- $0.00 / 1G
-
2022-05-11
- CAS:881681-00-1
- Min. Order: 1G
- Purity: 99%HPLC
- Supply Ability: 100KG
- Vonoprazan
-

- $0.00 / 1Kg/Bag
-
2021-10-23
- CAS:881681-00-1
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 100kg
Related articles - Uses and Mechanism of Vonoprazan
- Vonoprazan (trade name Takecab) is a first-in-class potassium-competitive acid blocker. It was approved in the Japanese market....
- May 10,2022
|
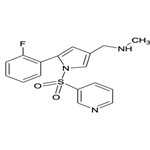
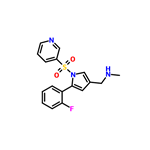
| Product Name: | Vonoprazan | | Synonyms: | TAK-438 (free base);Vonoprazan;1H-Pyrrole-3-methanamine,5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl);Vonoprazan-025;Voronazan interfluoroisomer;Vonoprazan related Impuirty 29; Vonoprazan m-Fluoro Isomer;Vonopraza/Vonoprazan fumarate | | CAS: | 881681-00-1 | | MF: | C17H16FN3O2S | | MW: | 345.39 | | EINECS: | | | Product Categories: | API | | Mol File: | 881681-00-1.mol | 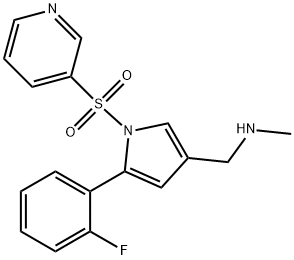 |
| | Vonoprazan Chemical Properties |
| Boiling point | 530.3±60.0 °C(Predicted) | | density | 1.31±0.1 g/cm3(Predicted) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | 9.06±0.10(Predicted) | | form | Solid | | color | Pale Yellow to Light Yellow | | Stability: | Hygroscopic | | InChI | InChI=1S/C17H16FN3O2S/c1-19-10-13-9-17(15-6-2-3-7-16(15)18)21(12-13)24(22,23)14-5-4-8-20-11-14/h2-9,11-12,19H,10H2,1H3 | | InChIKey | BFDBKMOZYNOTPK-UHFFFAOYSA-N | | SMILES | N1(S(C2=CC=CN=C2)(=O)=O)C(C2=CC=CC=C2F)=CC(CNC)=C1 | | CAS DataBase Reference | 881681-00-1 |
| | Vonoprazan Usage And Synthesis |
| Indications and Uses |
Vanoprazan fumarate is a new oral gastric acidity drug developed by Takeda Pharmaceutical and Otsuka Pharmaceuticals. It is used to treat duodenal ulcers, gastric ulcers, reflux esophagitis, gastric ulcers or recurrent duodenal ulcers caused by low dosages of Aspirin, and Helicobacter pylori. It can also supplement treatment of gastric ulcers, duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, early gastric cancer, and Helicobacter pylori infection gastritis.
Compared to traditional irreversible proton pump inhibitors (omeprazole, esomeprazole, etc.), Takecab has the following advantages:
1. Rapid effects, having the most drastic acid-suppressing effects on the first day of ingestion.
2. Oral intake, efficacy unaffected by gastric acid, does not require enteric administration.
Alleviated nighttime acid reflux.
| | Mechanisms of Action | Takecab (TAK-438) is a kind of potassium ion (K+) competitive acid blocker (P-CAB) and a reversible proton pump inhibitor. After this product enters the body, at the last step of gastric parietal cell acid secretion, it inhibits K+ ions from bonding with H+-K+-ATP enzymes (proton pump), thus stopping gastric acid secretion. It has a strong, lasting gastric acid suppression effect. TAK-438 is not mainly metabolized by protein CYP2C19, and it does not need to be activated by acid to inhibit protein pumps. As the drug enters the stomach at a high concentration, it will have the strongest suppressing effect at its first dosage, an effect which can last for up to 24 hours. TAK-438 is a stable acid, its formula is readily available and does not require optimized formulation (e.g. enteric coating), and its effective dosage amount does not vary dramatically between different patients.
| | Clinical Research | Compared to the traditional protein pump inhibitor Lansoprazole, Vonoprazan takes effect through competitive and reversible inhibition of K+ in protein pumps. Clinical and animal experiments show that Vonoprazan acts faster than PPI or H2 receptor blockers, has a stronger pH raising effect, can swiftly alleviate gastric symptoms, allows enzyme recovery after dissociation, and has few side effects. Multiple clinical trials have proved that in cases of erosive esophagitis, Vonoprazan prevents and treats gastric and duodenal ulcers. As a first-line response for eradicating helicobacter pylori, it has shown significant efficacy, higher than Lansoprazole and with minimal side effects.
Vonoprazan has high lipophilicity and dissociation constant, so in acidic environments, it can take effect without the activation of acid. Vonoprazan’s inhibition of protein pumps does not require acid activation. Upon entering the stomach at a high concentration in its first dosage, it produces its strongest inhibiting effect, which can last for 24 hours. Vonoprazan is a stable acid, and it can rapidly increase the pH in the stomach and suppress gastric acid.
Vonoprazan has minimal influence on other enzyme and bodily functions, making it very safe and tolerable. Traditional PPIs are metabolized by CYP2C19, while Vonoprazan is not. Its efficacy and required dosage do not differ dramatically in different patients, making it an advantageous individualized drug regimen for patients.
| | Patents | Chemical compound patent: 200680040789.7, application date: August 29, 2006, expiration date: August 29, 2026, legal status: holding rights.
Process patent: 201080018114.9, application date: February 24, 2013, expiration date: February 24, 2030. Legal status: In review – trial.
Composition patent: WO2014003199, application date: June 26, 2013, no Chinese patent.
| | Uses | Vonoprazan is used to treat an infection caused by the bacteria H. pylori. This bacteria can cause stomach/intestinal ulcers and irritation/swelling of the lining of the stomach. It may also increase the risk of stomach cancer. Treating the infection will help the ulcers get better and reduces the risk of serious damage to the lining of the stomach/intestines (such as bleeding, tearing).Vonoprazan is an acid blocker. It works by blocking acid production in the stomach. Decreasing excess stomach acid can help ulcers heal. Amoxicillin is an antibiotic used to treat a wide variety of bacterial infections (including H. pylori). Treating the infection helps the ulcers heal and reduces the risk of ulcers returning. Amoxicillin is a penicillin-type antibiotic. It works by stopping the growth of bacteria. Amoxicillin treats only bacterial infections. It will not work for viral infections (such as common cold, flu). | | Definition | ChEBI: Vonoprazan is a member of pyrroles. | | Side effects | Vonoprazan may cause any of the following side effects: diarrhoea, constipation, nausea, stomach discomfort, bloatedness, changes in taste, and rash. Some side effects may need immediate medical help. |
| | Vonoprazan Preparation Products And Raw materials |
|