- Trimetazidine
-

- $1.00 / 1g
-
2019-12-25
- CAS:5011-34-7
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/T
|
| Product Name: | Trimetazidine | | Synonyms: | ART-CHEM-BB B014361;ART-CHEM-BB B006602;AKOS B006602;1-[(2,3,4-trimethoxyphenyl)methyl]piperazine;1-(2,3,4-TRIMETHOXY-BENZYL)-PIPERAZINE;TRIMETAZIDINE;TRIMETAZIDINE HCL;TIMTEC-BB SBB007020 | | CAS: | 5011-34-7 | | MF: | C14H22N2O3 | | MW: | 266.34 | | EINECS: | 225-690-2 | | Product Categories: | | | Mol File: | 5011-34-7.mol | 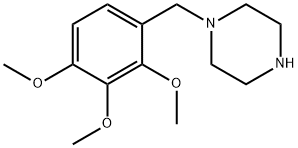 |
| | Trimetazidine Chemical Properties |
| Boiling point | bp2 200-205° | | density | 1.092±0.06 g/cm3(Predicted) | | vapor pressure | 0.003Pa at 25℃ | | storage temp. | Hygroscopic, Refrigerator, Under inert atmosphere | | solubility | Chlorofrom (Slightly), Methanol (Slightly) | | form | Solid | | pka | 9.07±0.10(Predicted) | | color | Colourless to Pale Yellow Gel | | Stability: | Hygroscopic | | LogP | 1.143 at 25℃ and pH6 | | CAS DataBase Reference | 5011-34-7(CAS DataBase Reference) |
| Hazard Codes | Xi | | HazardClass | IRRITANT |
| | Trimetazidine Usage And Synthesis |
| Outline | Trimetazidine (TMZ) is a piperazine derivative. Its trade name is Vasorel. Its chemical name is 1(2, 3, 4trimethoxy benzyl) piperazine dihydrochloride. It is an anti angina drug. It has the effect of antagonizing adrenaline, norepinephrine and vasopressin. It can relax vascular smooth muscle directly, reduce vascular resistance, increase blood flow of coronary and peripheral circulation, promote myocardial metabolism and production of myocardial energy. At the same time, it can reduce the oxygen consumption of the myocardium, thereby improving the balance between supply and demand of heart muscle oxygen. It can increase the tolerance to cardiac glycosides, increase the tolerance to exercise test in patients with angina pectoris, reduce the frequency of angina pectoris, and reduce the dosage of nitroglycerin. It is used in the treatment of coronary insufficiency, angina pectoris and old myocardial infarction. It can be used in combination with digitalis in patients with severe heart failure.
| | Mechanism of action | The effect of trimetazidine on the heart may be direct cell protection. It can prevent the decrease of ATP level in cells by conserving energy metabolism in ischemic oxygen cells. While maintaining the stability of the intracellular environment, it ensures the function of the ion pump and the normal operation of the trans membrane sodium potassium pump, reduces intracellular acidosis and prevents the accumulation of sodium and calcium within the cardiomyocytes, protects the cell contraction function and limits the cell dissolution and intimal damage caused by oxygen free radicals.
| | Synthetic method | The 194g six water piperazine and 260mL methanol are put into the reaction bottle, and the methyl formate is slowly added to the methyl formate. After the addition of 60mL, stir it for 30min, reflue for 5h, cool itto room temperature, get it decompressed with distillation, and got compound formyl piperazine. The 57g compound formyl piperazine and 70mL trimethoxy benzaldehyde are put into the reaction bottle and stirred until it has been completely dissolved and heated to 100 degrees. Slowly adding 20mL of formic acid with a mass fraction of 98%, heating up to 100~110℃ with reaction of 2h. After the reaction has been finished, a sodium bicarbonate solution with a mass fraction of 20% is added. The reflux goes on for 2h before heated and cooled. Then get it extracted with toluene (200mL * 3) and combined with toluene layer. Add 200mL water to it and mix with hydrochloric acid to adjust ph to 5~6. Add 200mL of sodium hydroxide solution with a mass fraction of 10%. After stirring 10min, the toluene layer is collected. A compound of trimetazidine is obtained by drying for 4h of toluene layer with anhydrous ammonium sulfate.
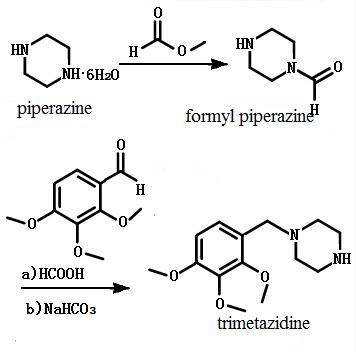
Figure 1 Synthesis route of trimetazidine | | Pharmacokinetics | The absorption of trimetazidine is rapid after oral administration. After single oral administration of trimetazidine 20mg, after 1.8h it will reach the peak plasma concentration and the plasma concentration peak is 53.6μg•L-1. After trimetazidine 20mg, Po, bid * 15d, the plasma concentration will reach a peak of 84.8μg•L-1. After oral administration of trimetazidine 20mg, the area under the concentration time curve can reach 508.9μg•h•L-1. When 20mg and bid were used for a long time, the area under the concentration time curve will reach 31.4μg•h•L-1. The bioavailability of trimetazidine is high, up to 88.7%. The protein binding rate of trimetazidine is about 16%, and the plasma volume is 318.6L. Trimetazidine T1/2 is 6h and 80% of the drug is excreted from the kidneys (62% of them are prototypes). The total scavenging rate of trimetazidine is 37.45L•h-1.
| | Pharmacodynamics | It has a strong anti angina effect, which is slower than nitroglycerin but has a longer duration. It has the effects of adrenalin, norepinephrine and vasopressin. It can reduce vascular resistance, increase coronary blood flow and peripheral blood flow, promote myocardial metabolism and improve myocardial energy production. At the same time, it can reduce the workload of the heart, reduce the oxygen consumption of the myocardium and the energy consumption of the myocardium, thereby improving the balance between supply and demand of heart muscle oxygen. It can increase the tolerance to cardiac glycosides.
| | Clinical application | It can be used for coronary artery insufficiency, angina pectoris, and old myocardial infarction. For patients with severe heart failure, it can be used with digitalis. Trimetazidine can be used alone in patients with mild angina pectoris. Trimetazidine can be used as an adjuvant in combination with other anti myocardial ischemia drugs in patients with moderate or severe angina pectoris. Single use of trimetazidine alone can reduce the frequency of angina pectoris and the dosage of nitroglycerin in patients with angina pectoris. In combination with other drugs, trimetazidine can relieve symptoms in patients with angina pectoris treated with other anti myocardial ischemia drugs. It is observed that trimetazidine combined with isosorbide dinitrate (30 mg. d-1) is effective in treating angina pectoris patients when 120mg•d-1cis not well controlled. In a group of patients with poor treatment with diltiazem (180 mg. d-1) or adrenaline beta blockers, the combination of trimetazidine can significantly reduce the frequency of angina pectoris and the dosage of nitroglycerin.
| | Adverse reaction | A few patients have gastrointestinal discomfort (nausea and vomiting).
| | Precaution | It is forbidden for patients with allergy and new myocardial infarction. For safety reasons, pregnant women and lactating women should be cautious in the use of this drug.
| | Originator | Vastarel,Biopharma,France,1963 | | Uses | 1-(2,3,4-Trimethoxybenzyl)piperazine is used in combination with atorvastatin in the treatment of coronary heart disease and angina pectoris. | | Definition | ChEBI: 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine is an aromatic amine. | | Manufacturing Process | Monoformylpiperazine is reacted molecule for molecule with 2,3,4-
trimethoxybenzyl chloride in the presence of 1 1/2 molecules of sodium
carbonate and in suspension in ethyl alcohol, during 2 to 3 hours.
The reaction product is filtered and the filtrate is evaporated in vacuo to
remove the alcohol. There remains an oily product from which the excess
formyl-ethylenediamine is removed by distillation under 1 mm Hg pressure up
to 125°C. The dark yellow, residual product is treated with 10% hydrochloric
acid at 100°C for 12 hours to eliminate the formyl group; it is evaporated to a
syrupy consistency and taken up with ethyl alcohol at the boiling point until
complete miscibility is attained; it is then discolored over carbon, filtered and
stored at low temperature.
The (2,3,4-trimethoxyphenyl) methylpiperazine hydrochloride precipitates as
white needles: the precipitate is drained and washed with anhydrous sulfuric
ether. Melting point: 222°C to 226°C. | | Therapeutic Function | Coronary vasodilator |
| | Trimetazidine Preparation Products And Raw materials |
|