Exemestane: A Breakthrough Aromatase Inhibitor Treatment for Advanced Breast Cancer
Feb 7,2024
General Description
Exemestane, a potent aromatase inhibitor, effectively reduces estrogen levels in postmenopausal women with breast cancer, showcasing high specificity for the aromatase enzyme without significantly impacting other steroid metabolism-related enzymes. Its pharmacokinetic profile indicates rapid absorption and dose-proportional pharmacokinetics, with food intake enhancing its absorption. Extensively metabolized, exemestane achieves significant estrogen suppression, crucial for treating hormone-sensitive cancers. Clinical trials, including phase II and a comparative phase III trial against megestrol, demonstrate exemestane's efficacy in inducing objective responses and disease stabilization in patients who have progressed on tamoxifen or failed multiple hormonal therapies, highlighting its value as a second-line therapy with superior outcomes in terms of response rates, disease progression, and survival times.

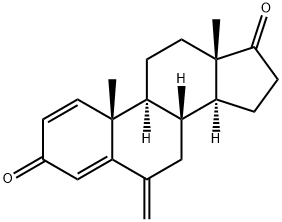
Figure 1. Exemestane
Pharmacodynamic Profile
Exemestane has been studied for its effectiveness in inactivating the aromatase enzyme, crucial for estrogen production, both in vitro and in vivo. These studies reveal exemestane's potent and specific action against aromatase, demonstrating a competitive mechanism where it binds irreversibly to the enzyme, leading to its inactivation through a suicide inhibition process. Notably, exemestane shows a high specificity for the aromatase enzyme without significantly affecting other enzymes or receptors related to steroid metabolism. This specificity is evident in its lack of effect on testosterone 5α-reductase, desmolase activity, and minimal affinity for androgen and estrogen receptors. Clinically, exemestane has proven effective in reducing estrogen levels significantly in postmenopausal women with a history of breast tumors, achieving maximum suppression with a 25mg dose. This suppression is crucial for managing conditions like advanced breast cancer, especially in patients who have not responded to previous therapies like tamoxifen. Exemestane's ability to reduce estrogen levels by up to 98% highlights its potential in treating hormone-sensitive cancers. Furthermore, exemestane's administration is effective both orally and subcutaneously, with notable antitumor effects observed in animal models of postmenopausal endocrine-responsive breast cancer. Despite its potent anti-estrogenic effects, exemestane does not significantly alter most other endocrine variables, although it can affect levels of certain hormones like DHEAS and SHBG in a dose-dependent manner. 1
Pharmacokinetic Profile
Exemestane, an aromatase inhibitor used in the treatment of breast cancer, exhibits a pharmacokinetic profile that has been thoroughly studied in healthy postmenopausal women, including those with a history of breast cancer. The drug is available in sugar-coated tablets and hard-gelatin capsules, both forms showing bioequivalence in terms of absorption rates and extents. Exemestane is absorbed rapidly into the bloodstream, reaching peak plasma concentrations (Cmax) within 1 to 2 hours post-administration. A single oral dose of 25mg after a meal results in a Cmax of 17.7 µg/L, and its pharmacokinetics are dose-proportional across the therapeutic range. However, at supratherapeutic doses, exemestane exhibits nonlinear pharmacokinetics. The area under the plasma concentration-time curve (AUC) demonstrates linear pharmacokinetics at therapeutic doses, indicating that the drug's absorption increases proportionally with the dose. Food intake significantly enhances the absorption of exemestane, increasing the plasma AUC by about 40% compared to the fasted state, although this does not affect its efficacy in inhibiting estrone sulphate. Exemestane undergoes extensive metabolism, involving oxidation and reduction processes, alongside hydrolysis and conjugation reactions leading to various metabolites. After administration, the drug and its metabolites are excreted equally through urine and feces over a period of 168 hours. The terminal elimination half-life of exemestane is approximately 24 hours, indicating that its plasma concentrations decline polyexponentially after reaching peak levels. 2
Therapeutic Trials
Exemestane has been evaluated for its efficacy in treating postmenopausal women with advanced breast cancer through various clinical trials, including phase II and a significant phase III trial comparing it to megestrol. These studies primarily focused on patients who either progressed or relapsed on antiestrogens like tamoxifen or were refractory to multiple hormonal therapies. In phase II trials, exemestane showed promising results. For patients previously treated with tamoxifen, exemestane achieved objective responses in 23 to 28% of cases, with an additional 19 to 24% experiencing disease stabilization for 24 weeks or more. The median time to response was around 16 weeks, with a median time to progression between 24 to 25 weeks. For those who had failed multiple hormonal therapies, exemestane induced objective responses in 7 to 26% of patients and disease stabilization in another 11 to 19%, showcasing its potential even in difficult-to-treat scenarios. The phase III trial highlighted exemestane's superiority over megestrol as a second-line therapy. Exemestane led to slightly higher objective response rates and long-term disease stabilization compared to megestrol, with a notably longer overall success period and a significantly extended time to disease progression. Additionally, exemestane demonstrated prolonged survival times, particularly notable in patients with predominant visceral disease, achieving better response rates in lung and liver lesions than megestrol. These findings underscore exemestane's effectiveness as a viable treatment option for postmenopausal women with advanced breast cancer, offering hope for improved outcomes even after other therapies have failed. 3
Reference
1. di-Salle E, Briatico G, Giudici D, et al. Novel aromatase and 5 α-reductase inhibitors. J Steroid Biochem Mol Biol. 1994; 49: 289-294.
2. Poggesi I, Jannuzzo MG, di-Salle E, et al. Effect of food and formulation on the pharmacokinetics (PK) and pharmacodynamics (PD) of a single oral dose of exemestane (Aromasin, EXE) [abstract no. 741]. Proc Am Soc Clin Oncol. 1999; 18: 193a.
3. Scott LJ, Wiseman LR. Exemestane. Drugs. 1999; 58(4):675-682.
- Related articles
- Related Qustion
- Exemestane used to treat breast cancer Jan 11, 2023
Exemestane is an oral steroidal aromatase inhibitor used to treat hormone responsive breast cancers in women who are postmenopausal - have gone through menopause.
- The Applications and Pharmacological effects of Exemestane Aug 20, 2019
Exemestane is the generic name for the trade name drug Aromasin?. In some cases, health care professionals may use the trade name Aromasin? when referring to the generic drug name exemestane. Exemestane is an anti-cancer hormone therapy. It is classified as an "aromatase inhibitor."
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCabozantinib effectively improves survival in renal cell carcinoma and hepatocellular carcinoma, offering benefits over alternatives despite manageable grade 3-4 adverse events.....
Feb 7,2024APIExemestane
107868-30-4You may like
- Exemestane
-
- 2025-12-04
- CAS:107868-30-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Exemestane
-

- $0.00 / 1kg
- 2025-12-04
- CAS:107868-30-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- Exemestane(Aromasin)
-

- $10.00 / 1box
- 2025-12-04
- CAS:107868-30-4
- Min. Order: 1box
- Purity: 99.99%
- Supply Ability: 100000box