Introduction of Melanotan I
Nov 24,2021
General description
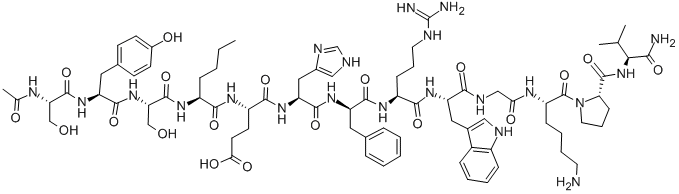
Melanotan I(Afamelanotide) is a first-in-class, synthetic, 13-amino acid peptide analogue of the endogenous alpha melanocyte-stimulating hormone (α-MSH). It differs structurally from its endogenous counterpart by only two amino acids - these structural differences improve biological efficacy by imparting a greater affinity for its target and a longer biological half-life. Afamelanotide is currently the only approved drug therapy used in the management of erythropoietic protoporphyria, having received approval in the EU in December 2014 and subsequent FDA approval in October 2019. Despite its relatively recent approval, afamelanotide has been available for use as an orphan drug in both the US and EU since 2008.
Application
1.According to different applications, it mainly includes the following aspects: nutrition, muscle strengthening and black beauty. Melanotan I is a laboratory made chemical similar to hormones in the human body. Melanotan I has been shown to have melanin production (tanning) and aphrodisiac effects on humans. Bioactive chemicalbook Melanotan (MT) II is a synthetic melanocortin receptor agonist. It is an injectable polypeptide hormone that promotes tanning.
Melanotan I is a peptide hormone, which belongs to α- A peptide hormone that mimics melanocyte stimulating hormone (alpha MSH). It can give you bronze chemicalbook color skin, and does not need sunlight exposure, so as to avoid the risk of skin sunburn and skin cancer. It can also enhance sexual desire. It is very suitable for competition preparation.
2.At present, three main formulations, Melanotan I (afamelan-otide), Melanotan II, and bremelanotide, exist. Afamelanotide, the first regulated a-MSG analogue, stimulates melanogenesis (pigmentation of the hair and skin in mammals) by heightening the production of eumelanin. Research continues on its use for vitiligo, solar urticarial, polymorphous light eruption, and prevention of squamous cell carcinoma and actinic keratoses in photosensitive subjects and organ-transplant recipients. Since afamelanotide was developed in the 1980s, a surge in demand for sunless-tanning agents has resulted in the marketing of multiple unregulated a-MSH analogues. Melanotan I was the first of these unregulated analogues with reported structure similar to afamelanotide, and with names often used interchangeably
Toxicity
Melanotan use and associated clinical outcomes:The review yielded eighteen clinical trials and twenty-one clinical case presentations. Side effects observed include nausea, darkening of existing naevi and yawning. Systemic toxidrome and melanoma have also been evidenced. Potential harms include bloodborne virus, infection and contaminated and mislabelled products. Shortcomings in clinical reporting have limited determinations of causality in diagnoses. Conclusion:Side effects observed in clinical trials are largely minor. Long-term health outcomes are as yet undocumented. Much of the harms related to melanotan use are associated with online sourcing of unregulated products.
The counterfeit PIEDs market and polypharming practices amongst users must be considered in reports of harm. Implications:This review makes recommendations to inform enhanced clinical responses, and has under scored the need for future Internet and clinical research to investigate prevalence and user profiling, and to map health outcomes in melanotan users.
Four adult patients developed various toxicities following treatment with afamelanotide for generalised vitiligo. They were part of a clinical trial looking at the efficacy and safety of narrowband UV-B (NB-UV-B) phototherapy and afamelanotide compared with NB-UV-B monotherapy. After one month of NB-UV-B phototherapy, they started receiving a series of four monthly implants of afamelanotide 16mg administered subcutaneously.
Storage
1.Keep in dark place,Sealed in dry,Store in freezer, under -20°C
2.Each drug shall be stored in cold storage (2-10 ℃), cool storage (below 20 ℃) or normal temperature storage (0-30 ℃) according to the storage conditions indicated on the drug label. The relative humidity of each warehouse shall be kept between 45% - 75%. The temperature range required by the cold storage, shade storage and normal temperature storage shall be based on the principle of ensuring the quality of drugs and meeting the storage conditions specified for drugs
Reference
1.Brennan R., Wells J. G. & Van Hout M. C., "An unhealthy glow? A review of melanotan use and associated clinical outcomes," Performance Enhancement & Health, Vol.3, No.2(2014), pp.78-92.
2. "Reactions 1491, p6 - 8 Mar 2014,".
- Related articles
- Related Qustion
- What is the difference between Melanotan-1 and Melanotan-2? Dec 6, 2024
Melanotan-1 and Melanotan-2 are 2 synthetic Melanotan molecules.
- Melanotan 1: mechanisms of action, clinical applications and safety Dec 8, 2023
Melanotan 1, a synthetic analogue of α-MSH, promotes tanning and potentially treating skin conditions. Lack of regulatory approval and safety concerns necessitate professional consultation before use.
Carbimazole is a member of the class of imidazoles that is methimazole in which the nitrogen bearing a hydrogen is converted into its ethoxycarbonyl derivative. A prodrug for methimazol,....
Nov 24,2021DrugsBoldenone is a DEA Schedule III controlled substance. Substances in the DEA Schedule III have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low phys....
Nov 25,2021DrugsMelanotan-1
75921-69-6You may like
- Melanotan 1
-

- $1.00 / 1g
- 2025-12-14
- CAS:75921-69-6
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Melanotan I
-

- $0.00 / 1g
- 2025-12-13
- CAS:75921-69-6
- Min. Order: 1g
- Purity: 98%min
- Supply Ability: 1000g
- Melanotan-1
-

- $0.00 / 1BOX
- 2025-12-11
- CAS:75921-69-6
- Min. Order: 1BOX
- Purity: 99%
- Supply Ability: 10000