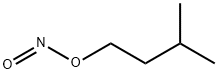
Synthesis and reactions of Isoamyl nitrite
Dec 10,2021
Isoamyl nitrite is a nitrite ester having isopentyl as the alkyl group. It has a role as a vasodilator agent and an antihypertensive agent. It derives from an isoamylol.

Properties
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet.It is also referred to as banapple gas.It was first documented in 1844 and came into medical use in 1867.
Uses
Amyl nitrite is employed medically to treat heart diseases as well as angina.
Amyl nitrite is sometimes used as an antidote for cyanide poisoning.It can act as an oxidant, to induce the formation of methemoglobin. Methemoglobin in turn can sequester cyanide as cyanomethemoglobin.
Amyl nitrite is used as a cleaning agent and solvent in industrial and household applications. It replaced dichlorodifluoromethane, an industrial chemical universally banned in 1996 due to damage to the ozone layer,as a printed circuit board cleaner.[citation needed] Trace amounts are added to some perfumes.
It is also used recreationally as an inhalant drug that induces a brief euphoric state, and when combined with other intoxicant stimulant drugs such as cocaine or MDMA, the euphoric state intensifies and is prolonged. Once some stimulative drugs wear off, a common side effect is a period of depression or anxiety, colloquially called a "come down"; amyl nitrite is sometimes used to combat these negative after-effects. This effect, combined with its dissociative effects, has led to its use as a recreational drug (see poppers).
Synthesis and reactions
Alkyl nitrites are prepared by the reaction of alcohols with nitrous acid:
ROH + HONO → RONO + H2O, where R = alkyl group
The reaction is called esterification. Synthesis of alkyl nitrites is, in general, straightforward and can be accomplished in home laboratories. A common procedure includes the dropwise addition of concentrated sulfuric acid to a cooled mixture of an aqueous sodium nitrite solution and an alcohol. The intermediately-formed stoichiometric mixture of nitrogen dioxide and nitric oxide then converts the alcohol to the alkyl nitrite, which, due to its low density, will form an upper layer that can be easily decanted from the reaction mixture.
Isoamyl nitrite decomposes in the presence of base to give nitrite salts and the isoamyl alcohol:
C5H11ONO + NaOH → C5H11OH + NaNO2
Amyl nitrite, like other alkyl nitrites, reacts with carbanions to give oximes.
Amyl nitrites are also useful as reagents in a modification of the Sandmeyer reaction. The reaction of the alkyl nitrite with an aromatic amine in a halogenated solvent produces a radical aromatic species, this then frees a halogen atom from the solvent. For the synthesis of aryl iodides diiodomethane is used,whereas bromoform is the solvent of choice for the synthesis of aryl bromides.
- Related articles
- Related Qustion
- The brief introduction of Isoamyl nitrite Aug 5, 2024
Isoamyl nitrite (amyl nitrite) is an intermediate for perfume preparation. It is an ingredient of “room odorizers.”
- Preparation method and application of isoamyl nitrite Apr 22, 2022
This article describes a background overview and preparation and use of Isoamyl nitrite
- Uses of Amyl nitrite Nov 10, 2021
Amyl nitrite had been used clinically as early as 1867, when the Scottish physician Sir Thomas Brunton used it as a vasodilator as treatment for angina pectoris in his patients. In the late 1880s, a protective effect on cyanide toxicity in
Hyaluronic acid abbreviated HA; conjugate base hyaluronate), also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycan....
Dec 10,2021Organic ChemistryMaleic anhydride appears as colorless crystalline needles, flakes, pellets, rods, briquettes, lumps or a fused mass. Melts at 113°F. Shipped both as a solid and in the molten state. Vapors, fumes and dusts strong irritate the eyes, skin and....
Dec 10,2021Carboxylic acids and derivativesIsoamyl nitrite
110-46-3You may like
- Isopentyl nitrite
-

- $200.00 / 1kg
- 2023-06-26
- CAS:110-46-3
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 500kg/month
- Isoamyl nitrite
-

- $25.00 / 1KG
- 2023-03-27
- CAS:110-46-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 23t
- Isoamylnitrite
-

- $100.00 / 1KG
- 2021-07-09
- CAS:110-46-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 40 Ton/Tons per Month