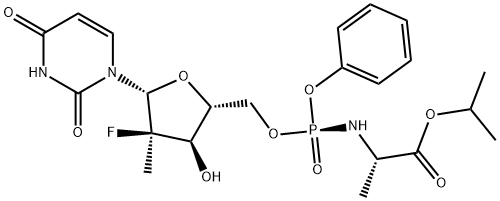
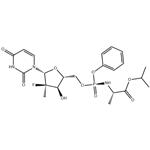
The review of sofosbuvir
Feb 23,2022
Background
Sofosbuvir, a prodrug metabolized to the active antiviral drug 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate, is currently in Phase 3 In clinical trials, it is used to treat hepatitis C. Studies have shown that sofosbuvir is a nucleotide inhibitor of hepatitis C virus and has a selective inhibitory effect on HCV NS5B polymerase. Oral treatment of hepatitis C drugs. Sofosbuvir, also known as sofosbuvir, sofosbuvir, is the world's first oral treatment of hepatitis C drugs, and is one of the most important new drugs approved in the United States in 2013. In the treatment of chronic hepatitis C, it can eliminate the need for the traditional injectable drug interferon (IFN). It was successfully developed by Gilead Sciences, the world's largest anti-HIV drug manufacturer. Peak sales could exceed $10 billion. As the picture 1 showed, there are sofosbuvir tablets.
Picture 1 Sofosbuvir tablets.
Pharmacology of sofosbuvir
Sofosbuvir (SOF) is the first nucleoside polymerase inhibitor, which was first launched in the United States in 2013. After sofosbuvir is absorbed, it is first metabolized into uracil triphosphate analogs (uridine triphosphate analogs) in the liver, and the uracil triphosphate analogs can be incorporated into the HCV RNA chain to interact with NS5B required for hepatitis C virus replication. The polymerase binds competitively, terminating the elongation of the viral RNA peptide chain.
Sofosbuvir is a "pan-genotype" anti-hepatitis C virus drug, which not only has inhibitory effect on genotype 1 hepatitis C virus, but also is effective against other genotypes of hepatitis C virus infection. It can also be used without interferon, creating a precedent for the treatment of hepatitis C without interferon. In addition, sofosbuvir is different from protease inhibitors, and the virus is not easy to develop resistance to it; even if resistance develops, the drug-resistant virus disappears quickly after the drug is stopped, and another drug combination can be replaced, and the sofosbuvir can be used again. Fesbuvir is used to re-treat patients who have failed previous treatments, hence the name "higher barrier to resistance" drugs.
Clinical use of sofosbuvir
For patients with genotype 2 hepatitis C virus infection, the "sustained virological response" rate of 12-week treatment with sofosbuvir + ribavirin can reach 93% to 98%; for patients with genotype 3 hepatitis C virus infection, sofosbuvir + ribavirin The efficacy of ribavirin is lower than that of patients with genotype 2 hepatitis C virus infection, and the "sustained virological response" rate can reach 92% to 96% after the course of treatment is extended to 24 weeks; for newly treated genotypes 1, 4, 5 or 6 The "sustained virological response" rate of sofosbuvir + pegylated interferon (Peg-IFN) combined with ribavirin in HCV-infected patients can reach more than 90%. However, for patients with genotype 1 hepatitis C virus infection, the sofosbuvir combined with ribavirin regimen, even if the course of treatment is extended to 24 weeks, its efficacy is lower than the triple therapy of sofosbuvir + Peg-IFNa combined with ribavirin plan.
In recent years, with more direct antiviral drugs on the market, sofosbuvir has gradually gotten rid of the combination of interferon or/and ribavirin with more side effects, and the new generation of direct antiviral drugs for the treatment of hepatitis C. Combined, the efficacy is better and safer. For example: Sofosbuvir can be combined with simeprevir to treat HCV genotypes 1 and 4; it can also be combined with ledipasvir to treat HCV genotypes other than genotypes 2 and 3 Viral infection; can also be combined with daclatasvir for the treatment of hepatitis C virus infection of all genotypes. These new treatment options allow people with HCV to receive safer and more effective treatment. However, the rate of "sustained virological response" in patients with cirrhosis is lower than in patients without cirrhosis, so the course of treatment in patients with cirrhosis needs to be prolonged or combined with ribavirin.
Renference
- Related articles
- Related Qustion
- Sofosbuvir: Characteristics and Pharmacokinetics Nov 22, 2024
Sofosbuvir is an effective, orally administered antiviral for hepatitis C with high absorption, favorable pharmacokinetics, and renal excretion.
- Sofosbuvir: Indications, Mechanism of Action and Side Effects Aug 16, 2024
Sofosbuvir is a direct-acting antiviral drug primarily used in combination with other drugs to treat hepatitis C virus (HCV) infection.
Uridine is a substance naturally produced by the liver and can be classified as a nucleoside.....
Feb 23,2022APIParacetamol (acetaminophen) was first used in 1893 and is the only remaining p-aminophenol available in clinical practice.....
Feb 23,2022Antipyretic analgesicsSofosbuvir
1190307-88-0You may like
- Sofosbuvir
-
- 2025-12-14
- CAS:1190307-88-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sofosbuvir
-

- $0.00 / 1KG
- 2025-12-13
- CAS:1190307-88-0
- Min. Order: 1KG
- Purity: 98%-102%
- Supply Ability: 100kgs
- Sofosbuvir
-

- $1.00 / 1kg
- 2025-12-11
- CAS:1190307-88-0
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 1000